Pediatr Gastroenterol Hepatol Nutr.
2016 Mar;19(1):1-11. 10.5223/pghn.2016.19.1.1.
Cholestasis beyond the Neonatal and Infancy Periods
- Affiliations
-
- 1Department of Medical Education, Johns Hopkins All Children's Hospital, St. Petersburg, FL, United States.
- 2Department of Gastroenterology and Nutrition, Johns Hopkins All Children's Hospital, St. Petersburg, FL, United States. mwilsey1@jhmi.edu
- KMID: 2160470
- DOI: http://doi.org/10.5223/pghn.2016.19.1.1
Abstract
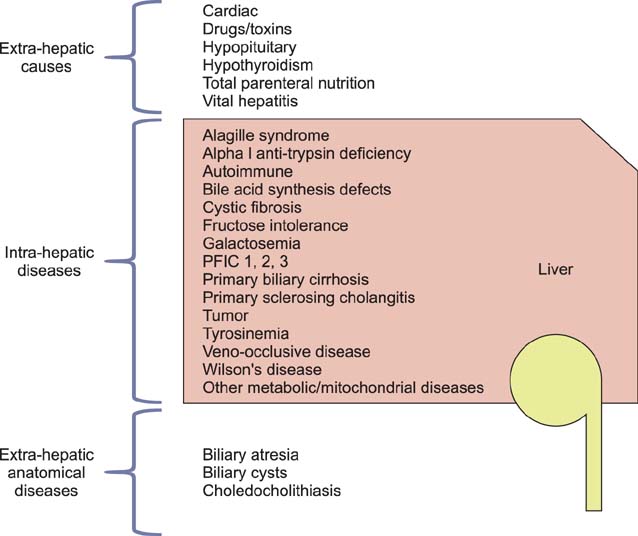
- Cholestasis results from impairment in the excretion of bile, which may be due to mechanical obstruction of bile flow or impairment of excretion of bile components into the bile canaliculus. When present, cholestasis warrants prompt diagnosis and treatment. The differential diagnosis of cholestasis beyond the neonatal period is broad and includes congenital and acquired etiologies. It is imperative that the clinician differentiates between intrahepatic and extrahepatic origin of cholestasis. Treatment may be supportive or curative and depends on the etiology. Recent literature shows that optimal nutritional and medical support also plays an integral role in the management of pediatric patients with chronic cholestasis. This review will provide a broad overview of the pathophysiology, diagnostic approach, and management of cholestasis beyond the neonatal and infancy periods.
Keyword
MeSH Terms
Figure
Reference
-
1. Suchy FJ, Sokol RJ, Balistreri WF. Liver disease in children. New York: Cambridge University Press;2014.2. Kremer AE, Bolier R, van Dijk R, Oude Elferink RP, Beuers U. Advances in pathogenesis and management of pruritus in cholestasis. Dig Dis. 2014; 32:637–645.
Article3. Chappell LC, Gurung V, Seed PT, Chambers J, Williamson C, Thornton JG. PITCH Study Consortium. Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: semifactorial randomised clinical trial. BMJ. 2012; 344:e3799.
Article4. Kronsten V, Fitzpatrick E, Baker A. Management of cholestatic pruritus in paediatric patients with alagille syndrome: the King's College Hospital experience. J Pediatr Gastroenterol Nutr. 2013; 57:149–154.
Article5. Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, et al. North American Society for Pediatric Gastroenterology. Hepatology and Nutrition. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004; 39:115–128.
Article6. Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991; 14:551–566.
Article7. Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: Structure-activity relationships. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G290–G297.
Article8. Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009; 51:565–580.
Article9. Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998; 339:1217–1227.
Article10. Stringer MD, Taylor DR, Soloway RD. Gallstone composition: are children different? J Pediatr. 2003; 142:435–440.11. Friesen CA, Roberts CC. Cholelithiasis. Clinical characteristics in children. Case analysis and literature review. Clin Pediatr (Phila). 1989; 28:294–298.12. Katsika D, Grjibovski A, Einarsson C, Lammert F, Lichtenstein P, Marschall HU. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology. 2005; 41:1138–1143.
Article13. O'Neill JA Jr. Choledochal cyst. Curr Probl Surg. 1992; 29:361–410.14. Shah OJ, Shera AH, Zargar SA, Shah P, Robbani I, Dhar S, et al. Choledochal cysts in children and adults with contrasting profiles: 11-year experience at a tertiary care center in Kashmir. World J Surg. 2009; 33:2403–2411.
Article15. Singham J, Yoshida EM, Scudamore CH. Choledochal cysts: part 2 of 3: Diagnosis. Can J Surg. 2009; 52:506–511.16. Panagiotakaki E, Tzetis M, Manolaki N, Loudianos G, Papatheodorou A, Manesis E, et al. Genotype-phenotype correlations for a wide spectrum of mutations in the Wilson disease gene (ATP7B). Am J Med Genet A. 2004; 131:168–173.
Article17. Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol. 2009; 19:231–234.
Article18. Schilsky ML, Ala A. Genetic testing for Wilson disease: availability and utility. Curr Gastroenterol Rep. 2010; 12:57–61.
Article19. Ranucci G, Socha P, Iorio R. Wilson disease: what is still unclear in pediatric patients? Clin Res Hepatol Gastroenterol. 2014; 38:268–272.
Article20. Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014; 4:25–36.
Article21. Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2012; 36:Suppl 1. S26–S35.
Article22. Jansen PL, Müller MM. Progressive familial intrahepatic cholestasis types 1, 2, and 3. Gut. 1998; 42:766–767.
Article23. Alagille D, Habib EC, Thomassin N. L'atresie des voies biliaires intrahepatiques avec voies biliaires extrahepatiques permeables chez l'enfant. Paris: Flammarion;1969. p. 301–318.24. Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet. 2003; 40:891–895.
Article25. Davis AR, Rosenthal P, Escobar GJ, Newman TB. Interpreting conjugated bilirubin levels in newborns. J Pediatr. 2011; 158:562–565.e1.
Article26. Weiss JS, Gautam A, Lauff JJ, Sundberg MW, Jatlow P, Boyer JL, et al. The clinical importance of a protein-bound fraction of serum bilirubin in patients with hyperbilirubinemia. N Engl J Med. 1983; 309:147–150.
Article27. Harb R, Thomas DW. Conjugated hyperbilirubinemia: screening and treatment in older infants and children. Pediatr Rev. 2007; 28:83–91.
Article28. Ovchinsky N, Moreira RK, Lefkowitch JH, Lavine JE. Liver biopsy in modern clinical practice: a pediatric point-of-view. Adv Anat Pathol. 2012; 19:250–262.29. Bucuvalas JC, Cutfield W, Horn J, Sperling MA, Heubi JE, Campaigne B, et al. Resistance to the growth-promoting and metabolic effects of growth hormone in children with chronic liver disease. J Pediatr. 1990; 117:397–402.
Article30. de Albuquerque Taveira AT, Fernandes MI, Galvão LC, Sawamura R, de Mello Vieira E, de Paula FJ. Impairment of bone mass development in children with chronic cholestatic liver disease. Clin Endocrinol (Oxf). 2007; 66:518–523.31. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012; 33:291–302.
Article32. Keil R, Snajdauf J, Rygl M, Pycha K, Kotalová R, Drábek J, et al. Diagnostic efficacy of ERCP in cholestatic infants and neonates--a retrospective study on a large series. Endoscopy. 2010; 42:121–126.
Article33. Bhavsar MS, Vora HB, Giriyappa VH. Choledochal cysts : a review of literature. Saudi J Gastroenterol. 2012; 18:230–236.
Article34. Lewis VA, Adam SZ, Nikolaidis P, Wood C, Wu JG, Yaghmai V, et al. Imaging of choledochal cysts. Abdom Imaging. 2015; 40:1567–1580.
Article35. Cai Q, Keilin S, Obideen K, Li Y. Intraduodenal hydrochloric acid infusion for facilitation of cannulation of the dorsal pancreatic duct at ERCP in patients with pancreas divisum: a preliminary study. Am J Gastroenterol. 2010; 105:1450–1451.
Article36. Ahrar H, Jafarpishe MS, Hekmatnia A, Solouki R, Emami MH. Magnetic resonance cholangiography compared with endoscopic retrograde cholangiography in the diagnosis of primary sclerosing cholangitis. J Res Med Sci. 2014; 19:1150–1154.37. Moff SL, Kamel IR, Eustace J, Lawler LP, Kantsevoy S, Kalloo AN, et al. Diagnosis of primary sclerosing cholangitis: a blinded comparative study using magnetic resonance cholangiography and endoscopic retrograde cholangiography. Gastrointest Endosc. 2006; 64:219–223.
Article38. Feldstein AE, Perrault J, El-Youssif M, Lindor KD, Freese DK, Angulo P. Primary sclerosing cholangitis in children: a long-term follow-up study. Hepatology. 2003; 38:210–217.
Article39. Eppens EF, van Mil SW, de Vree JM, Mok KS, Juijn JA, Oude Elferink RP, et al. FIC1, the protein affected in two forms of hereditary cholestasis, is localized in the cholangiocyte and the canalicular membrane of the hepatocyte. J Hepatol. 2001; 35:436–443.
Article40. European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson's disease. J Hepatol. 2012; 56:671–685.41. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006; 354:731–739.
Article42. Hegade VS, Kendrick SF, Jones DE. Drug treatment of pruritus in liver diseases. Clin Med (Lond). 2015; 15:351–357.
Article43. Chang Y, Golkar L. The use of naltrexone in the management of severe generalized pruritus in biliary atresia: report of a case. Pediatr Dermatol. 2008; 25:403–404.
Article44. Ständer S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol. 2003; 139:1463–1470.45. Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2003; 98:2736–2741.
Article46. Müller C, Pongratz S, Pidlich J, Penner E, Kaider A, Schemper M, et al. Treatment of pruritus in chronic liver disease with the 5-hydroxytryptamine receptor type 3 antagonist ondansetron: a randomized, placebo-controlled, double-blind cross-over trial. Eur J Gastroenterol Hepatol. 1998; 10:865–870.
Article47. Nightingale S, Ng VL. Optimizing nutritional management in children with chronic liver disease. Pediatr Clin North Am. 2009; 56:1161–1183.
Article48. Mager DR, Wykes LJ, Roberts EA, Ball RO, Pencharz PB. Branched-chain amino acid needs in children with mild-to-moderate chronic cholestatic liver disease. J Nutr. 2006; 136:133–139.
Article49. Shneider BL, Magee JC, Bezerra JA, Haber B, Karpen SJ, Raghunathan T, et al. Childhood Liver Disease Research Education Network (ChiLDREN). Efficacy of fat-soluble vitamin supplementation in infants with biliary atresia. Pediatrics. 2012; 130:e607–e614.
Article50. Shen YM, Wu JF, Hsu HY, Ni YH, Chang MH, Liu YW, et al. Oral absorbable fat-soluble vitamin formulation in pediatric patients with cholestasis. J Pediatr Gastroenterol Nutr. 2012; 55:587–591.
Article51. Jensen M, Abu-El-Haija M, Bishop W, Rahhal RM. Difficulty achieving vitamin D sufficiency with high-dose oral repletion therapy in infants with cholestasis. J Pediatr Gastroenterol Nutr. 2015; 61:187–189.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Beta Thalassemia Presenting with Neonatal Cholestasis and Extensive Hemosiderosis: A Case Report
- Evaluation of the Underlying Etiology and Long-Term Prognostic Factors in Neonatal Cholestasis
- A Case of Neonatal Cholestasis Associated with Congenital Adrenal Hyperplasia
- A Case of Idiopathic Congenital Neonatal Cholestasis in a Patient with Down Syndrome
- Outcome of Alagille Syndrome Patients Who Had Previously Received Kasai Operation during Infancy: A Single Center Study