Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study
- Affiliations
-
- 1Unit of General Surgery I, Papa Giovanni XXIII Hospital, Bergamo, Italy. marco.ceresoli89@gmail.com
- 2Unit of General, Emergency and Transplant Surgery, Sant'Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy.
- 3Unit of Gynecologic Surgery, Papa Giovanni XXIII Hospital, Bergamo, Italy.
- 4Unit of Gynaecology, Jena University Hospital, Jena, Germany.
- 5University of Hawaii at Manoa, Honolulu, HI, USA.
- 6Unit of Gynecologic Oncology, Sant'Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy.
- KMID: 2158803
- DOI: http://doi.org/10.3802/jgo.2015.26.1.54
Abstract
OBJECTIVE
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been recently reported with favorable oncological outcomes as treatment of advanced epithelial ovarian cancer (EOC). The aim of this study was to demonstrate the feasibility of CRS+HIPEC with cisplatin and paclitaxel for the treatment of advanced EOC.
METHODS
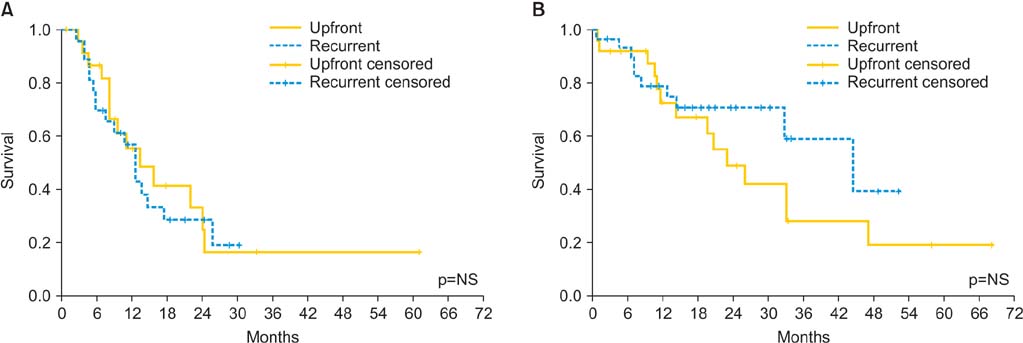
This is a prospective observational study of 54 patients, from April 2007 to October 2013, with primary or recurrent peritoneal carcinomatosis due to EOC. The mean age was 54.51+/-9.34. Thirty patients (59%) had primary EOC, and 24 patients (41%) had recurrent disease.
RESULTS
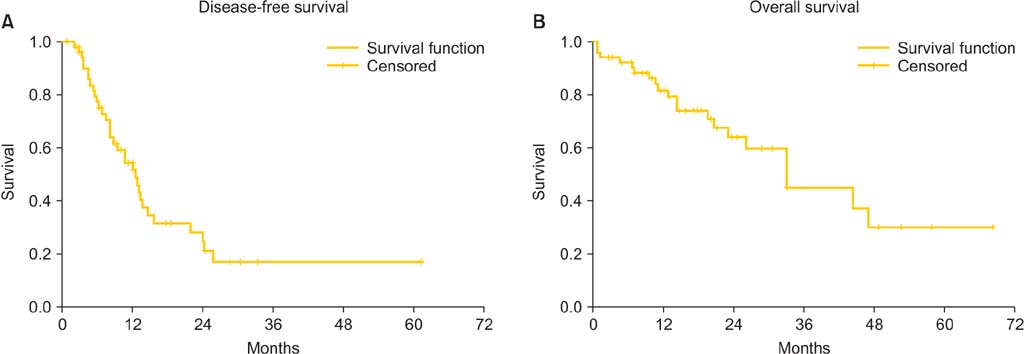
Mean peritoneal cancer index was 10.11 (range, 0 to 28), complete cytoreduction (CC0) was achieved for 47 patients (87%), CC1 for seven patients (13%). Patients with suboptimal cytoreduction (CC2 and CC3) were not included in the study. The mean stay in intensive care unit was 4.73+/-5.51 days and the mean hospitalization time was 24.0+/-10.03 days. We did not observe any intraoperative death. Seven patients (13%) required additional operations. Three patients (5.6%) died within 30 days from the procedure. Severe complications were seen in 19 patients (35.2%). During the follow-up period, disease recurred in 33 patients (61.1%); the median disease-free survival time was 12.46 months and the median overall survival time was 32.91 months.
CONCLUSION
CRS+HIPEC with cisplatin and paclitaxel for advanced EOC is feasible with acceptable morbidity and mortality. Additional follow-up and further studies are needed to determine the effects of HIPEC on long term survival.
MeSH Terms
-
Adult
Antineoplastic Combined Chemotherapy Protocols/administration & dosage/adverse effects/*therapeutic use
Cisplatin/administration & dosage/adverse effects
Combined Modality Therapy
Cytoreduction Surgical Procedures/adverse effects/methods
Feasibility Studies
Female
Humans
Hyperthermia, Induced/adverse effects/*methods
Infusions, Parenteral
Kaplan-Meier Estimate
Middle Aged
Neoplasm Recurrence, Local/drug therapy/surgery
Ovarian Neoplasms/*drug therapy/surgery
Paclitaxel/administration & dosage/adverse effects
Prospective Studies
Treatment Outcome
Cisplatin
Paclitaxel
Figure
Cited by 3 articles
-
Hyperthermic intraperitoneal chemotherapy in advanced ovarian cancer
Tao Wu, Xi-Xia Zhao, Guo-Qing Wang
J Gynecol Oncol. 2018;29(4):. doi: 10.3802/jgo.2018.29.e51.Effect of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on relapse pattern in primary epithelial ovarian cancer: a propensity score based case-control study
Marco Ceresoli, Apollonia Verrengia, Giulia Montori, Luisa Busci, Federico Coccolini, Luca Ansaloni, Luigi Frigerio
J Gynecol Oncol. 2018;29(3):. doi: 10.3802/jgo.2018.29.e53.Incorporation of paclitaxel-based hyperthermic intraperitoneal chemotherapy in patients with advanced-stage ovarian cancer treated with neoadjuvant chemotherapy followed by interval debulking surgery: a protocol-based pilot study
Yong Jae Lee, Jung-Yun Lee, Min-Soo Cho, Eun Ji Nam, Sang Wun Kim, Sunghoon Kim, Young Tae Kim
J Gynecol Oncol. 2019;30(1):. doi: 10.3802/jgo.2019.30.e3.
Reference
-
1. Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005; 55:10–30.2. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–3200.3. Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011; 21:750–755.4. Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996; 335:1950–1955.5. Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001; 19:1001–1007.6. Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006; 354:34–43.7. Chua TC, Robertson G, Liauw W, Farrell R, Yan TD, Morris DL. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol. 2009; 135:1637–1645.8. de Bree E, Romanos J, Michalakis J, Relakis K, Georgoulias V, Melissas J, et al. Intraoperative hyperthermic intraperitoneal chemotherapy with docetaxel as second-line treatment for peritoneal carcinomatosis of gynaecological origin. Anticancer Res. 2003; 23:3019–3027.9. Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, et al. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol. 2007; 106:193–200.10. North Bristol NHS Trust. WHO Performance status [Internet]. Bristol: North Bristol NHS Trust;2013. cited 2014 Oct 17. Available from: http://www.nbt.nhs.uk/sites/default/files/filedepot/incoming/WHO_Performance_Status.doc.11. Helm CW, Bristow RE, Kusamura S, Baratti D, Deraco M. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008; 98:283–290.12. Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009; 374:1371–1382.13. Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg. 1999; 384:576–587.14. Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996; 15:49–58.15. Sarnaik AA, Sussman JJ, Ahmad SA, McIntyre BC, Lowy AM. Technology for the delivery of hyperthermic intraoperative intraperitoneal chemotherapy: a survey of techniques. In : Gonzaalez-Moreno S, editor. Advances in peritoneal surface oncology. Berlin: Springer;2007. p. 75–82.16. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [Internet]. Bethesda, MD: Cancer Therapy Evaluation Program;c2006. cited 2014 Oct 17. Available from: http://www.eortc.be/services/doc/ctc/ctcaev3.pdf.17. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216.18. Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003; 361:2099–2106.19. Markman M, Rowinsky E, Hakes T, Reichman B, Jones W, Lewis JL Jr, et al. Phase I trial of intraperitoneal taxol: a Gynecoloic Oncology Group study. J Clin Oncol. 1992; 10:1485–1491.20. Hofstra LS, Bos AM, de Vries EG, van der Zee AG, Willemsen AT, Rosing H, et al. Kinetic modeling and efficacy of intraperitoneal paclitaxel combined with intravenous cyclophosphamide and carboplatin as first-line treatment in ovarian cancer. Gynecol Oncol. 2002; 85:517–523.21. Rossi CR, Mocellin S, Pilati P, Foletto M, Quintieri L, Palatini P, et al. Pharmacokinetics of intraperitoneal cisplatin and doxorubicin. Surg Oncol Clin N Am. 2003; 12:781–794.22. Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009; 249:900–907.23. Pomel C, Ferron G, Lorimier G, Rey A, Lhomme C, Classe JM, et al. Hyperthermic intra-peritoneal chemotherapy using oxaliplatin as consolidation therapy for advanced epithelial ovarian carcinoma: results of a phase II prospective multicentre trial. CHIPOVAC study. Eur J Surg Oncol. 2010; 36:589–593.24. Konigsrainer I, Horvath P, Struller F, Grischke EM, Wallwiener D, Konigsrainer A, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in recurrent epithelial ovarian cancer with peritoneal metastases: a single centre experience. Langenbecks Arch Surg. 2014; 399:589–594.25. Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363:943–953.26. Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013; 19:6979–6994.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for ovarian cancer
- A case of advanced ovarian cancer which was treated with topotecan after taxol-cisplatin treatment failed
- Incorporation of paclitaxel-based hyperthermic intraperitoneal chemotherapy in patients with advanced-stage ovarian cancer treated with neoadjuvant chemotherapy followed by interval debulking surgery: a protocol-based pilot study
- Intraperitoneal cisplatin chemotherapy for advanced ovarian cancer
- CHIPOR, HORSE, and beyond: unraveling the role of hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer