Yonsei Med J.
2008 Oct;49(5):851-852. 10.3349/ymj.2008.49.5.851.
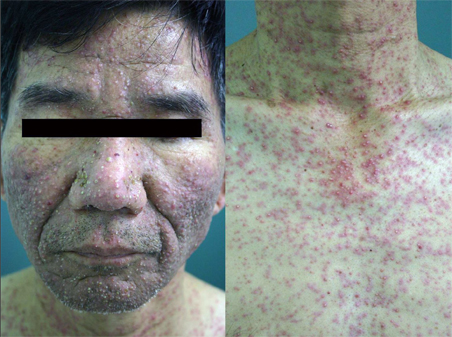
Severe Acneiform Eruption Induced by Cetuximab (Erbitux(R))
- Affiliations
-
- 1Yonsei Star Skin & Laser Clinic, Seoul, Korea. kwanglee@yuhs.ac
- 2Department of Dermatology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2158205
- DOI: http://doi.org/10.3349/ymj.2008.49.5.851
Abstract
- Epidermal growth factor has an important role in the regulation of proliferation and differentiation in epidermal keratinocytes, as well as in the survival, angiogenesis and metastasis of cancer cells. Cetuximab is a chimeric monoclonal antibody selective for the epidermal growth factor receptor that induces a broad range of cellular responses that enhance tumor sensitivity to radiotherapy and chemotherapeutic agents. However, it can cause adverse events in the patient including acneiform eruption, asthenia, abdominal pain and nausea/vomiting. We report a case of severe acneiform eruption induced by cetuximab in a 56-year-old man with colorectal cancer and liver metastases.
Keyword
MeSH Terms
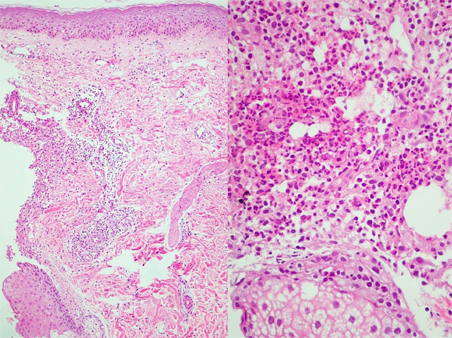
Figure
Reference
-
1. Reynolds NA, Wagstaff AJ. Cetuximab: in the treatment of metastatic colorectal cancer. Drugs. 2004. 64:109–118. discussion 119-21.2. Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005. 16:1425–1433.
Article3. Jacot W, Bessis D, Jorda E, Ychou M, Fabbro M, Pujol JL, et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol. 2004. 151:238–241.
Article4. Kimyai-Asadi A, Jih MH. Follicular toxic effects of chimeric anti-epidermal growth factor receptor antibody cetuximab used to treat human solid tumors. Arch Dermatol. 2002. 138:129–131.
Article5. Monti M, Mancini LL, Ferrari B, Rahal D, Santoro A. Complications of therapy and a diagnostic dilemma case. Case 2. Cutaneous toxicity induced by cetuximab. J Clin Oncol. 2003. 21:4651–4653.6. Segaert S, Tabernero J, Chosidow O, Dirschka T, Elsner J, Mancini L, et al. The management of skin reactions in cancer patients receiving epidermal growth factor receptor targeted therapies. J Dtsch Dermatol Ges. 2005. 3:599–606.
Article7. Molinari E, De Quatrebarbes J, André T, Aractingi S. Cetuximab-induced acne. Dermatology. 2005. 211:330–333.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acneiform Eruption Occurring after Injection of Cetuximab (Erbitux(R), IMC-C225)
- Two Cases of Acneiform Eruption Caused by Epidermal Growth Factor Receptor Inhibitors
- Clinical Features of the Cutaneous Adverse Events Induced by Combination Chemotherapy that Includes Cetuximab (Erbitux(R))
- Acneiform Eruption Induced by Radotinib (IY5511 : HCL)
- A Case of Hair Change and Acneiform Eruption Induced by ZD1839 (Iressa(R))