Yonsei Med J.
2007 Aug;48(4):561-572. 10.3349/ymj.2007.48.4.561.
Nanoparticles-A Thoracic Toxicology Perspective
- Affiliations
-
- 1MRC/University of Edinburgh Centre for Inflammation Research, ELEGI Colt Laboratory, Queen's Medical Research Institute, 47 Little France Crescent, Edinburgh, EH16 4TJ, Scotland, UK. rodger.duffin@ed.ac.uk
- KMID: 2158160
- DOI: http://doi.org/10.3349/ymj.2007.48.4.561
Abstract
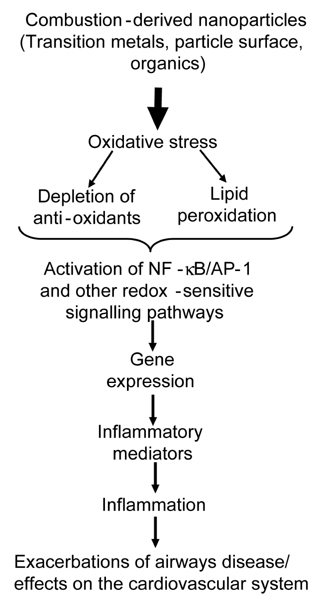
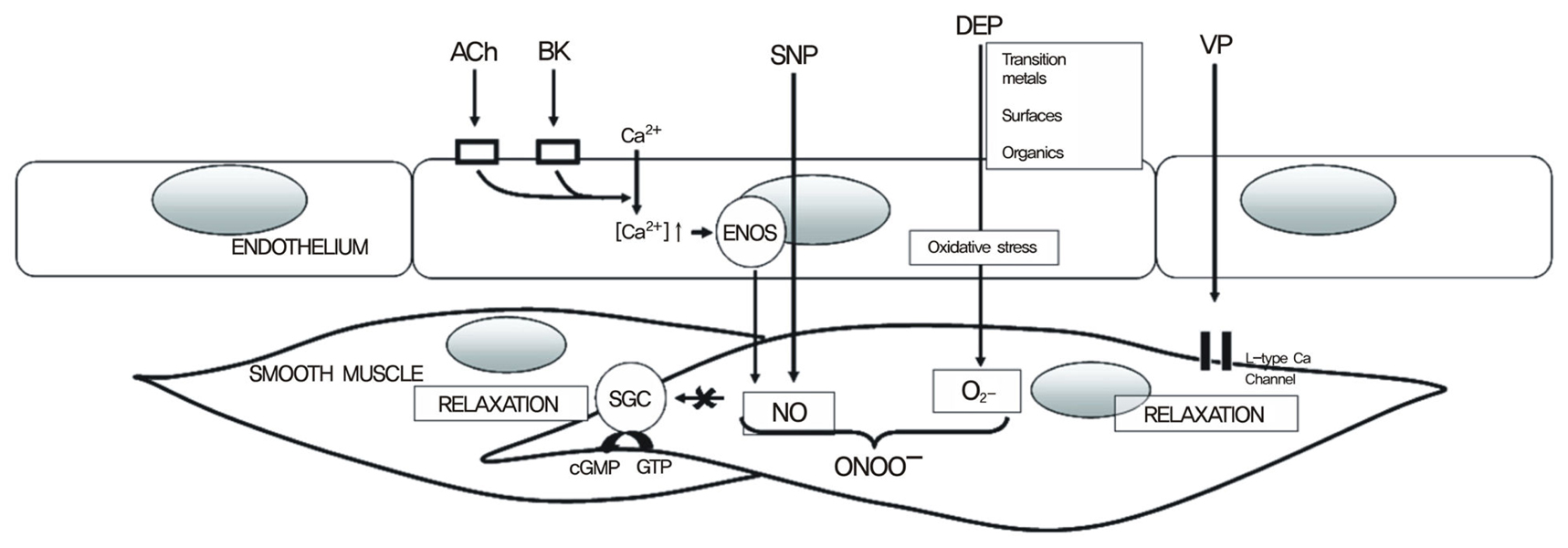
- A substantial literature demonstrates that the main ultrafine particles found in ambient urban air are combustion-derived nanoparticles (CDNP) which originate from a number of sources and pose a hazard to the lungs. For CDNP, three properties appear important-surface area, organics and metals. All of these can generate free radicals and so induce oxidative stress and inflammation. Inflammation is a process involved in the diseases exhibited by the individuals susceptible to the effects of PM- development and exacerbations of airways disease and cardiovascular disease. It is therefore possible to implicate CDNP in the common adverse effects of increased PM. The adverse effects of increases in PM on the cardiovascular system are well-documented in the epidemiological literature and, as argued above, these effects are likely to be driven by the combustion-derived NP. The epidemiological findings can be explained in a number of hypotheses regarding the action of NP:-1) Inflammation in the lungs caused by NP causes atheromatous plaque development and destabilization; 2) The inflammation in the lungs causes alteration in the clotting status or fibrinolytic balance favouring thrombogenesis; 3) The NP themselves or metals/organics released by the particles enter the circulation and have direct effects on the endothelium, plaques, the clotting system or the autonomic nervous system/ heart rhythm. Environmental nanoparticles are accidentally produced but they provide a toxicological model for a new class of purposely 'engineered' NP arising from the nanotechnology industry, whose effects are much less understood. Bridging our toxicological knowledge between the environmental nanoparticles and the new engineered nanoparticles is a considerable challenge.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Impact of Nanomaterials in Immune System
Jiyoung Jang, Dae-Hyoun Lim, In-Hong Choi
Immune Netw. 2010;10(3):85-91. doi: 10.4110/in.2010.10.3.85.
Reference
-
1. Royal Society and Royal Academy of Engineering. Nanoscience and nanotechnologies: opportunities and uncertainties. 2004. The Royal Society.2. Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995. 345:176–178.
Article3. Utell MJ, Frampton MW. Acute health effects of ambient air pollution: the ultrafine particle hypothesis. J Aerosol Med. 2000. 13:355–359.
Article4. Donaldson K, Tran L, Jimenez L, Duffin R, Newby DE, Mills N, et al. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005. 2:10.
Article5. Quality of Urban Air Review Group. Airborne particulate matter in the United Kingdom: third report of the Quality of Urban Air Review Group. Quality of Urban Air Review Group. 1996. Quality of Urban Air Review Group.6. Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002. 360:1233–1242.
Article7. Schlesinger RB, Cassee F. Atmospheric secondary inorganic particulate matter: the toxicological perspective as a basis for health effects risk assessment. Inhal Toxicol. 2003. 15:197–235.
Article8. Pope CA, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995. 151:669–674.
Article9. de Hartog JJ, Hoek G, Mirme A, Tuch T, Kos GP, ten Brink HM, et al. Relationship between different size classes of particulate matter and meteorology in three European cities. J Environ Monit. 2005. 7:302–310.
Article10. Air Quality Expert Group. Particulate matter in the United Kingdom. 2005. London: Published by the Department of Environment, Food and Rural Affairs.11. Elder A, Gelein R, Finkelstein J, Phipps R, Frampton M, Utell M, et al. On-road exposure to highway aerosols. 2. Exposures of aged, compromised rats. Inhal Toxicol. 2004. 16:Suppl 1. 41–53.
Article12. Kittelson DB, Watts WF, Johnson JP, Remerowki ML, Ische EE, Oberdorster G, et al. On-road exposure to highway aerosols. 1. Aerosol and gas measurements. Inhal Toxicol. 2004. 16:Suppl 1. 31–39.
Article13. Afshari A, Matson U, Ekberg LE. Characterization of indoor sources of fine and ultrafine particles: a study conducted in a full-scale chamber. Indoor Air. 2005. 15:141–150.
Article14. Dennekamp M, Howarth S, Dick CA, Cherrie JW, Donaldson K, Seaton A. Ultrafine particles and nitrogen oxides generated by gas and electric cooking. Occup Environ Med. 2001. 58:511–516.
Article15. Donaldson K, Jimenez LA, Rahman I, Faux SP, MacNee W, Gilmour PS, et al. Vallyathan V, Castranova V, Shi X, editors. Respiratory health effects of ambient air pollution particles: Role of reactive species. Oxygen/nitrogen radicals: lung injury and disease. 2004. New York: Marcel Dekker;257–288. (Vol 187 in Lung Biology in Health and Disease. Executive Editor: Lenfant C.).16. Donaldson K, Stone V, Borm PJ, Jimenez LA, Gilmour PS, Schins RP, et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic Biol Med. 2003. 34:1369–1382.
Article17. Miyabara Y, Yanagisawa R, Shimojo N, Takano H, Lim HB, Ichinose T, et al. Murine strain differences in airway inflammation caused by diesel exhaust particles. Eur Respir J. 1998. 11:291–298.
Article18. Tsurudome Y, Hirano T, Yamato H, Tanaka I, Sagai M, Hirano H, et al. Changes in levels of 8-hydroxyguanine in DNA, its repair and OGG1 mRNA in rat lungs after intratracheal administration of diesel exhaust particles. Carcinogenesis. 1999. 20:1573–1576.
Article19. Nordenhall C, Pourazar J, Blomberg A, Levin JO, Sandstrom T, Adelroth E. Airway inflammation following exposure to diesel exhaust: a study of time kinetics using induced sputum. Eur Respir J. 2000. 15:1046–1051.
Article20. Ichinose T, Yajima Y, Nagashima M, Takenoshita S, Nagamachi Y, Sagai M. Lung carcinogenesis and formation of 8-hydroxy-deoxyguanosine in mice by diesel exhaust particles. Carcinogenesis. 1997. 18:185–192.
Article21. Arimoto T, Yoshikawa T, Takano H, Kohno M. Generation of reactive oxygen species and 8-hydroxy-2'-deoxyguanosine formation from diesel exhaust particle components in L1210 cells. Jpn J Pharmacol. 1999. 80:49–54.
Article22. Bonvallot V, Baeza-Squiban A, Baulig A, Brulant S, Boland S, Muzeau F, et al. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am J Respir Cell Mol Biol. 2001. 25:515–521.
Article23. Hirano S, Furuyama A, Koike E, Kobayashi T. Oxidative-stress potency of organic extracts of diesel exhaust and urban fine particles in rat heart microvessel endothelial cells. Toxicology. 2003. 187:161–170.
Article24. Li N, Venkatesan MI, Miguel A, Kaplan R, Gujuluva C, Alam J, et al. Induction of heme oxygenase-1 expression in macrophages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element. J Immunol. 2000. 165:3393–3401.
Article25. Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001. 7:20–26.
Article26. McNeilly JD, Jimenez LA, Clay MF, MacNee W, Howe A, Heal MR, et al. Soluble transition metals in welding fumes cause inflammation via activation of NF-kappaB and AP-1. Toxicol Lett. 2005. 158:152–157.
Article27. Marano F, Boland S, Bonvallot V, Baulig A, Baeza-Squiban A. Human airway epithelial cells in culture for studying the molecular mechanisms of the inflammatory response triggered by diesel exhaust particles. Cell Biol Toxicol. 2002. 18:315–320.28. Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J Immunol. 1999. 163:5582–5591.29. Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Jibiki I, Takizawa H, et al. Diesel exhaust particles activate p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am J Respir Crit Care Med. 2000. 161:280–285.
Article30. Takizawa H, Ohtoshi T, Kawasaki S, Kohyama T, Desaki M, Kasama T, et al. Diesel exhaust particles induce NF-kappa B activation in human bronchial epithelial cells in vitro: importance in cytokine transcription. J Immunol. 1999. 162:4705–4711.31. Gilmour PS, Rahman I, Donaldson K, MacNee W. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol. 2003. 284:L533–L540.32. Terada N, Hamano N, Maesako KI, Hiruma K, Hohki G, Suzuki K, et al. Diesel exhaust particulates upregulate histamine receptor mRNA and increase histamine-induced IL-8 and GM-CSF production in nasal epithelial cells and endothelial cells. Clin Exp Allergy. 1999. 29:52–59.
Article33. Salvi SS, Nordenhall C, Blomberg A, Rudell B, Pourazar J, Kelly FJ, et al. Acute exposure to diesel exhaust increases IL-8 and GRO-alpha production in healthy human airways. Am J Respir Crit Care Med. 2000. 161:550–557.
Article34. Yang HM, Ma JY, Castranova V, Ma JK. Effects of diesel exhaust particles on the release of interleukin-1 and tumor necrosis factor-alpha from rat alveolar macrophages. Exp Lung Res. 1997. 23:269–284.
Article35. Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, et al. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp Lung Res. 1998. 24:85–100.
Article36. Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004. 109:2655–2671.37. Brook RD, Brook JR, Rajagopalan S. Air pollution: the "Heart" of the problem. Curr Hypertens Rep. 2003. 5:32–39.
Article38. Donaldson K, Mills N, MacNee W, Robinson S, Newby D. Role of inflammation in cardiopulmonary health effects of PM. Toxicol Appl Pharmacol. 2005. 207(2 Suppl):483–488.
Article39. Viles-Gonzalez JF, Anand SX, Valdiviezo C, Zafar MU, Hutter R, Sanz J, et al. Update in atherothrombotic disease. Mt Sinai J Med. 2004. 71:197–208.40. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002. 105:1135–1143.
Article41. Van Lente F. Markers of inflammation as predictors in cardiovascular disease. Clin Chim Acta. 2000. 293:31–52.
Article42. Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol. 2002. 39:935–942.
Article43. Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005. 294:3003–3010.
Article44. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999. 340:115–126.45. Blum A, Miller HI. The role of inflammation in atherosclerosis. Isr J Med Sci. 1996. 32:1059–1065.46. Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996. 97:1715–1722.
Article47. Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001. 164:1665–1668.
Article48. Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002. 65:1513–1530.
Article49. Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002. 65:1531–1543.
Article50. Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002. 105:411–414.
Article51. Mills N, Amin N, Robinson SD, Anand A, Davies J, Patel D, et al. Do Inhaled 99mTechnetium-labeled carbon nanoparticles do not translocate into the circulation in man. Am J Resp Crit Care Med. 2005. submitted.52. Khandoga A, Stampfl A, Takenaka S, Schulz H, Radykewicz R, Kreyling W, et al. Ultrafine particles exert prothrombotic but not inflammatory effects on the hepatic microcirculation in healthy mice in vivo. Circulation. 2004. 109:1320–1325.
Article53. Nemmar A, Nemery B, Hoet PH, Vermylen J, Hoylaerts MF. Pulmonary inflammation and thrombogenicity caused by diesel particles in hamsters: role of histamine. Am J Respir Crit Care Med. 2003. 168:1366–1372.
Article54. Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation. 2003. 107:1202–1208.
Article55. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980. 288:373–376.
Article56. Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986. 315:1046–1051.
Article57. Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996. 334:150–154.
Article58. Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999. 99:1411–1415.
Article59. Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000. 102:994–999.
Article60. van den Eijnden-Schrauwen Y, Kooistra T, de Vries RE, Emeis JJ. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 1995. 85:3510–3517.61. Brommer EJ. The level of extrinsic plasminogen activator (t-PA) during clotting as a determinant of the rate of fibrinolysis; inefficiency of activators added afterwards. Thromb Res. 1984. 34:109–115.
Article62. Fox KA, Robison AK, Knabb RM, Rosamond TL, Sobel BE, Bergmann SR. Prevention of coronary thrombosis with subthrombolytic doses of tissue-type plasminogen activator. Circulation. 1985. 72:1346–1354.
Article63. Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005. 112:3930–3936.
Article64. Bellmann B, Muhle H, Creutzenberg O, Mermelstein R. Irreversible pulmonary changes induced in rat lung by dust overload. Environ Health Perspect. 1992. 97:189–191.
Article65. Morrow PE. Dust overloading of the lungs: update and appraisal. Toxicol Appl Pharmacol. 1992. 113:1–12.
Article66. Mauderly JL, Cheng YS, Snipes MB. Particle overload in toxicological studies: friend or foe, and how can we know? J Aerosol Med. 1990. 3:Suppl 1. 169–187.67. Morrow PE. Possible mechanisms to explain dust overloading of the lungs. Fundam Appl Toxicol. 1988. 10:369–384.
Article68. Tran CL, Buchanan D, Cullen RT, Searl A, Jones AD, Donaldson K. Inhalation of poorly soluble particles. II. Influence Of particle surface area on inflammation and clearance. Inhal Toxicol. 2000. 12:1113–1126.
Article69. Cullen RT, Tran CL, Buchanan D, Davis JM, Searl A, Jones AD, et al. Inhalation of poorly soluble particles. I. Differences in inflammatory response and clearance during exposure. Inhal Toxicol. 2000. 12:1089–1111.
Article70. Driscoll KE, Carter JM, Howard BW, Hassenbein DG, Pepelko W, Baggs RB, et al. Pulmonary inflammatory, chemokine, and mutagenic responses in rats after subchronic inhalation of carbon black. Toxicol Appl Pharmacol. 1996. 136:372–380.
Article71. Oberdorster G, Ferin J, Soderholm S, Gelein R, Cox C, Baggs R, et al. Dogston J, McCallum RI, editors. Increased pulmonary toxicity of inhaled ultrafine particles: due to lung overload alone? Inhaled Part VII, Proceedings of the 7th International Symposium. 1991. 295–302.72. Gilmour PS, Ziesenis A, Morrison ER, Vickers MA, Drost EM, Ford I, et al. Pulmonary and systemic effects of short-term inhalation exposure to ultrafine carbon black particles. Toxicol Appl Pharmacol. 2004. 195:35–44.
Article73. Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004. 61:442–447.
Article74. Hohr D, Steinfartz Y, Schins RP, Knaapen AM, Martra G, Fubini B, et al. The surface area rather than the surface coating determines the acute inflammatory response after instillation of fine and ultrafine TiO2 in the rat. Int J Hyg Environ Health. 2002. 205:239–244.
Article75. Duffin R, Tran L, Brown D, Stone V, Donaldson K. Pro-inflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: highlighting the role of particle surface area and surface reactivity. Inhal Toxicol. 2007. 19:849–856.
Article76. Wilson MR, Lightbody JH, Donaldson K, Sales J, Stone V. Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicol Appl Pharmacol. 2002. 184:172–179.
Article77. Brown DM, Stone V, Findlay P, MacNee W, Donaldson K. Increased inflammation and intracellular calcium caused by ultrafine carbon black is independent of transition metals or other soluble components. Occup Environ Med. 2000. 57:685–691.
Article78. Beck-Speier I, Dayal N, Karg E, Maier KL, Schumann G, Schulz H, et al. Oxidative stress and lipid mediators induced in alveolar macrophages by ultrafine particles. Free Radic Biol Med. 2005. 38:1080–1092.
Article79. Stone V, Tuinman M, Vamvakopoulos JE, Shaw J, Brown D, Petterson S, et al. Increased calcium influx in a monocytic cell line on exposure to ultrafine carbon black. Eur Respir J. 2000. 15:297–303.
Article80. Brown DM, Donaldson K, Borm PJ, Schins RP, Dehnhardt M, Gilmour P, et al. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol. 2004. 286:L344–L353.81. Tamaoki J, Isono K, Takeyama K, Tagaya E, Nakata J, Nagai A. Ultrafine carbon black particles stimulate proliferation of human airway epithelium via EGF receptor-mediated signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L1127–L1133.
Article82. Timblin CR, Shukla A, Berlanger I, Berube KA, Churg A, Mossman BT. Ultrafine airborne particles cause increases in protooncogene expression and proliferation in alveolar epithelial cells. Toxicol Appl Pharmacol. 2002. 179:98–104.
Article83. Donaldson K, Tran CL. An introduction to the short-term toxicology of respirable industrial fibres. Mutat Res. 2004. 553:5–9.
Article84. Nyberg K, Johansson U, Rundquist I, Camner P. Estimation of pH in individual alveolar macrophage phagolysosomes. Exp Lung Res. 1989. 15:499–510.
Article85. Hesterberg TW, Miiller WC, Musselman RP, Kamstrup O, Hamilton RD, Thevenaz P. Biopersistence of man-made vitreous fibers and crocidolite asbestos in the rat lung following inhalation. Fundam Appl Toxicol. 1996. 29:267–279.
Article86. Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005. 207:221–231.
Article87. Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A. 2004. 67:87–107.
Article88. Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004. 77:126–134.
Article89. Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in Rats. Toxicol Sci. 2004. 77:117–125.
Article90. Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L698–L708.
Article91. Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicol Lett. 2005. 155:377–384.
Article92. Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003. 66:1909–1926.
Article93. Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005. 39:1378–1383.
Article94. Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006. 160:121–126.
Article95. Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005. 155:73–85.
Article96. Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005. 5:1676–1684.
Article97. Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett. 2006. 165:88–100.
Article98. Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005. 146:882–893.
Article99. Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. 2006. 70:129–140.
Article100. Don Porto CA, Hoet PH, Verschaeve L, Schoeters G, Nemery B. Genotoxic effects of carbon black particles, diesel exhaust particles, and urban air particulates and their extracts on a human alveolar epithelial cell line (A549) and a human monocytic cell line (THP-1). Environ Mol Mutagen. 2001. 37:155–163.
Article101. Schins RP. Mechanisms of genotoxicity of particles and fibers. Inhal Toxicol. 2002. 14:57–78.
Article102. de Kok TM, Hogervorst JG, Briede JJ, van Herwijnen MH, Maas LM, Moonen EJ, et al. Genotoxicity and physicochemical characteristics of traffic-related ambient particulate matter. Environ Mol Mutagen. 2005. 46:71–80.
Article103. Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: Mechanisms. Int J Cancer. 2004. 109:799–809.
Article104. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991. 11:81–128.
Article105. Chen M, von Mikecz A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Exp Cell Res. 2005. 305:51–62.
Article106. Geiser M, Rothen-Rutlshauser B, Kapp N, Schurch S, Kreyling W, Schulz H, et al. Ultrafine particles cross cellular membranes by non-phagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005. 113:1555–1560.
Article107. Dick CA, Brown DM, Donaldson K, Stone V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal Toxicol. 2003. 15:39–52.
Article108. Donaldson K, Beswick PH, Gilmour PS. Free radical activity associated with the surface of particles: a unifying factor in determining biological activity? Toxicol Lett. 1996. 88:293–298.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Photo-triggered Theranostic Nanoparticles in Cancer Therapy
- Accelerated ecotoxicity of photoreactive nanoparticles on Moina macrocopa
- Quercetin diminishes the apoptotic pathway of magnetite nanoparticles in rats' ovary: Antioxidant status and hormonal profiles
- The Trend of Organic Based Nanoparticles in the Treatment of Diabetes and Its Perspectives
- Nasal and Pulmonary Toxicity of Titanium Dioxide Nanoparticles in Rats