Yonsei Med J.
2005 Oct;46(5):679-686. 10.3349/ymj.2005.46.5.679.
The Regulators of VEGF Expression in Mouse Ovaries
- Affiliations
-
- 1Department of Obstetrics & Gynecology, Eulji University School of Medicine, Seoul, Korea. pwi3110@eulji.or.kr
- 2Department of Pathology, Eulji University School of Medicine, Seoul, Korea.
- 3Eulji Life Science Institute, Eulji University School of Medicine, Seoul, Korea.
- KMID: 2158149
- DOI: http://doi.org/10.3349/ymj.2005.46.5.679
Abstract
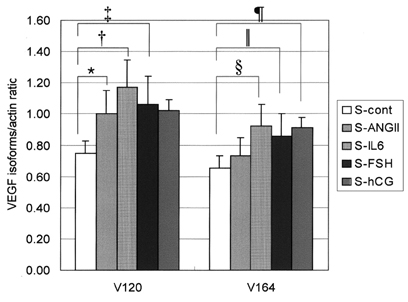
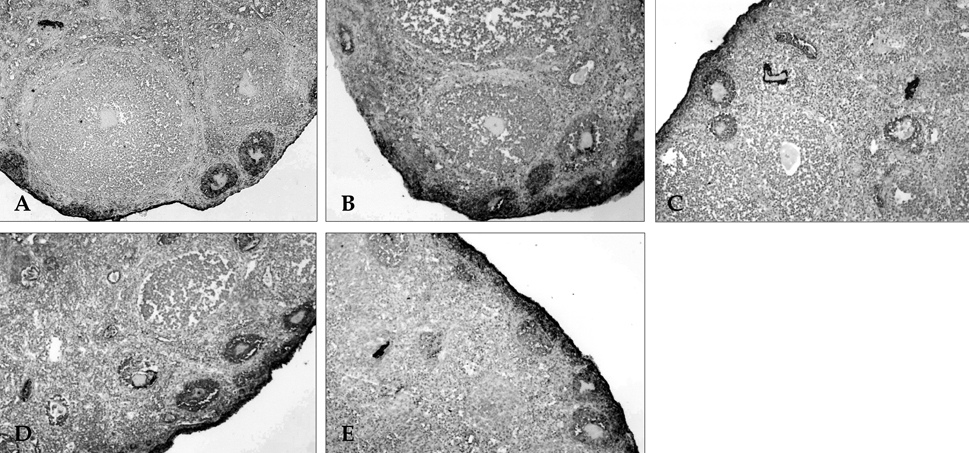
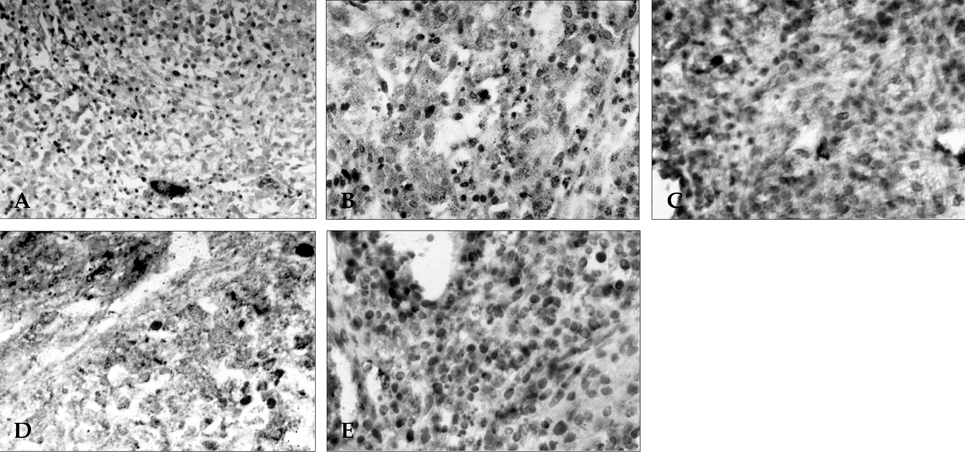
- The objectives of this study were to explore whether ovarian vascular endothelial growth factor (VEGF) expression in mice can be regulated by IL-6 (interleukin-6), angiotensin II, FSH, and hCG; and to test whether the mouse ovarian VEGF expression can result in angiogenesis. The ICR mice were sacrificed, and their ovaries were recovered. Recovered ovaries were treated with IL-6, angiotensin II, FSH, and hCG separately and incubated for 24 hours in alpha-MEM. Expression of mRNA and protein of VEGF were assessed by RT-PCR and immunohistochemistry. The resulting angiogenesis was evaluated through immunohistochemical analysis for CD34. Treatment of mice ovaries with IL-6, FSH, and hCG resulted in a significant increase of VEGF mRNA, and IL-6 was the most potent inducer of VEGF. IL-6 and FSH resulted in increased neovascularization in the follicular phase of mouse ovaries. In contrast, angiotensin II could not increase VEGF expression or neovascularization. We documented an in vitro increase in VEGF expression by IL-6, FSH, and hCG; and reaffirmed that the proliferative response of murine ovarian endothelial cells paralleled an increase of VEGF expression.
Keyword
MeSH Terms
-
Vascular Endothelial Growth Factor A/analysis/*genetics
RNA, Messenger/analysis
Ovary/*metabolism
Mice, Inbred ICR
Mice
Interleukin-6/pharmacology
Immunohistochemistry
Gene Expression Regulation/*drug effects
Follicle Stimulating Hormone/pharmacology
Female
Chorionic Gonadotropin/pharmacology
Antigens, CD34/analysis
Animals
Figure
Cited by 1 articles
-
IGF-1 Counteracts TGF-β-Mediated Enhancement of Fibronectin for in Vitro Human Lens Epithelial Cells
So-Hyang Chung, Sun-Ah Jung, Young Jae Cho, Joon H. Lee, Eung Kweon Kim
Yonsei Med J. 2007;48(6):949-954. doi: 10.3349/ymj.2007.48.6.949.
Reference
-
1. Geva E, Jaffe RB. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril. 2000. 74:429–438.2. Wulff C, Wiegand SJ, Saunders PT, Scobie GA, Fraser HM. Angiogenesis during follicular development in primate and its inhibition by treatment with truncated Flt-1-Fc. (vascular endothelial growth factor Trap(A40)). Endocrinology. 2001. 142:3244–3254.3. Redmer DA, Reynolds LP. Angiogenesis in the ovary. Rev Reprod. 1996. 1:182–192.4. Christenson LK, Stouffer RL. Follicle stimulating hormone and luteinizing hormone/chorionic gonadotropin stimulation of vascular endothelial growth factor production by macaque granulosa cells from pre- and periovulatory follicles. J Clin Endocrinol Metab. 1997. 82:2135–2142.5. Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996. 271:736–741.6. Hayashi K, Miyamoto A, Berisha B, Kosmann MR, Okuda K, Schams D. Regulation of angiotensin II production and angiotensin receptors in microvascular endothelial cells from bovine corpus luteum. Biol Reprod. 2000. 62:162–167.7. Song KH, Song J, Jeong GB, Kim JM, Jung SH, Song J. Vascular endothelial growth factor- its relation to neovascularization and their significance as prognostic factors in renal cell carcinoma. Yonsei Med J. 2001. 42:539–546.8. Stehouwer CD, Lambert J, Donker AJ, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc Res. 1997. 34:55–68.9. Koch AE. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998. 41:951–962.10. Kang SM, Kwon HM, Hong BK, Kim D, Kim IJ, Choi EY, et al. Expression of leptin receptor (Ob-R) in human atherosclerotic lesions: potential role intimal neovascularization. Yonsei Med J. 2000. 41:68–75.11. Anteby EY, Hurwitz A, Korach O, Revel A, Simon A, Finci-Yeheskel Z, et al. Human follicular nitric oxide pathway: relationship to follicular size, oestradiol concentrations and ovarian blood flow. Hum Reprod. 1996. 11:1947–1951.12. Stouffer RL, Martinez-Chequer JC, Molskness TA, Xu F, Hazzard TM. Regulation and action of angiogenic factors in the primate ovary. Arch Med Res. 2001. 32:567–575.13. Battaglia C, Genazzani AD, Regnani G, Primavera MR, Petraglia F, Volpe A. Perifollicular Doppler flow and follicular fluid vascular endothelial growth factor concentrations in poor responders. Fertil Steril. 2000. 74:809–812.14. Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci USA. 1990. 87:3092–3096.15. Wang TH, Horng SG, Chang CL, Wu HM, Tsai YJ, Wang HS, et al. Human Chorionic gonadotropin-induced ovarian hyperstimulation syndrome is associated with up-regulation of vascular endothelial growth factor. J Clin Endocrinol Metab. 2002. 87:3300–3308.16. Anasti JN, Kalantaridou SN, Kimzey LM, George M, Nelson LM. Human follicle fluid vascular endothelial growth factor concentrations are correlated with luteinization in spontaneously developing follicles. Hum Reprod. 1998. 13:1144–1147.17. Anand RJ, Paust HJ, Altenpohl K, Mukhopadhyay AK. Regulation of vascular endothelial growth factor production by Leydig cells in vitro: the role of protein kinase A and mitogen-activated protein kinase cascade. Biol Reprod. 2003. 68:1663–1673.18. Imthurn B, Cox SL, Jenkin G, Trounson AO, Shaw JM. Gonadotrophin administration can benefit ovarian tissue grafted to the body wall: implications for human ovarian grafting. Mol Cell Endocrinol. 2000. 163:141–146.19. Einspanier R, Schnfelder M, Muller K, Stojkovic M, Kosmann M, Wolf E, et al. Expression of the vascular endothelial growth factor and its receptors and effects of VEGF during in vitro maturation of bovine cumulusoocyte complexes(COC). Mol Reprod Dev. 2002. 62:29–36.20. Williams B, Baker AQ, Gallacher B, Lodwick D. Angiotensin II increases vascular permeability factor gene expression by human vascular smooth muscle cells. Hypertension. 1995. 25:913–917.21. Tamarat R, Silvestre JS, Duriez M, Levy BI. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor and inflammation related pathways. Lab Invest. 2002. 82:747–756.22. Kotani E, Sugimoto M, Kamata H, Fujii N, Saitoh M, Usuki S, et al. Biological roles of angiotensin II via its type 2 receptor during rat follicle atresia. Am J Physiol. 1999. 276:E25–E33.23. Yoshimura Y, Karube M, Aoki H, Oda T, Koyama N, Nagai A, et al. Angiotensin II induces ovulation and oocyte maturation in rabbit ovaries via the AT2 receptor subtype. Endocrinology. 1996. 137:1204–1211.24. Gaytan F, Morales C, Garcia-Pardo L, Reymundo C, Bellido C, Sanchez-Criado JE. A quantitative study of changes in the human corpus luteum microvasculature during the menstrual cycle. Biol Reprod. 1999. 60:914–919.25. Shimizu T, Jiang JY, Iijima K, Miyabayashi K, Ogawa Y, Sasada H, et al. Induction of follicular development by direct single injection of vascular endothelial growth factor gene fragments into the ovary of miniature gilts. Biol Reprod. 2003. 69:1388–1393.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of c-Kit/SCF and Inhibin-alpha in Ovarian Follicles During Mouse Development
- Expression of Vascular Endothelial Growth Factor in Human Ovary
- Expression of SDF-1alpha and leptin, and their effect on expression of angiogenic factors in mouse ovaries
- Transcription factors in the maintenance and survival of primordial follicles
- The Prognostic Effect of VEGF Expression in Squamous Cell Carcinoma of the Cervix Treated with Radiation Therapy Alone