Yonsei Med J.
2005 Feb;46(1):141-148. 10.3349/ymj.2005.46.1.141.
Impact of Cyclosporin on Podocyte ZO-1 Expression in Puromycin Aminonucleoside Nephrosis Rats
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Institute of Kidney Disease, Yonsei University College of Medicine, Seoul, Korea. docbsk@medimail.co.kr
- KMID: 2158124
- DOI: http://doi.org/10.3349/ymj.2005.46.1.141
Abstract
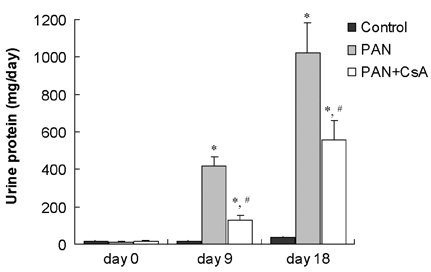
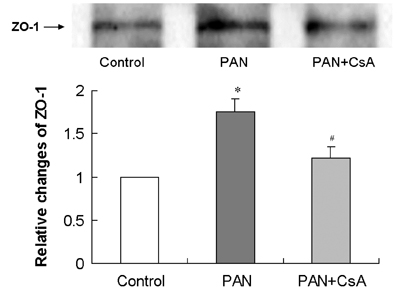
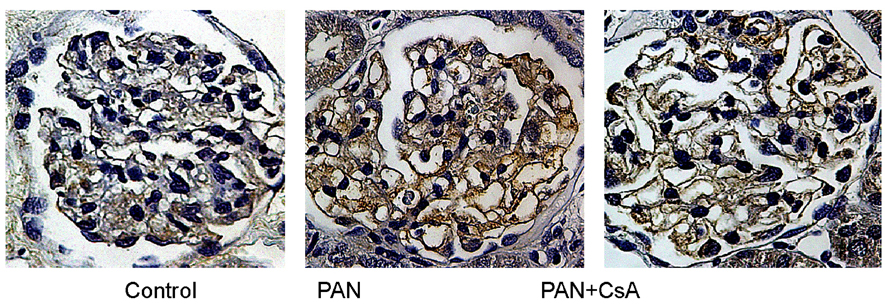
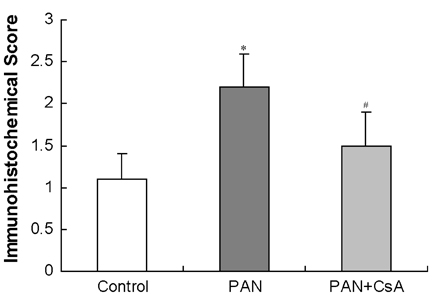
- Puromycin aminonucleoside (PAN) -induced nephrosis is a well-described model of human idiopathic nephrotic syndrome, but the mechanism of PAN's effect is not completely understood. To investigate whether proteinuria in the PAN model is associated with an alteration of zonula occludens-1 (ZO-1) expression within the glomeruli, and whether cyclosporin A (CsA) has an effect on proteinuria and ZO-1 expression in this model, eighteen Sprague Dawley (SD) rats were assigned into three groups. Twelve rats received a single intraperitoneal injection of PAN (15 mg/100 g). The other six rats received an equal volume of saline (normal control group; control). CsA solution was administered intraperitoneally once a day for 20 days after the PAN injection (n=6, PAN+CsA). The remaining six rats received PAN, but they didn't receive CsA (n=6, PAN). Compared to control rats (35.1 +/- 5.4 mg/day), the 24-hour urinary protein excretion on day 18 was significantly higher in the PAN rats (1021.9 +/- 128.9 mg/day, p< 0.01), and the CsA treatment partly reversed the increase in proteinuria in the PAN rats (556.4 +/- 102.3 mg/day, p< 0.05). Glomerular ZO-1 protein expressions were significantly increased in the PAN rats as compared to the control group on day 20 (176%, p< 0.01). CsA treatment for 20 days in the PAN rats inhibited the increase in ZO-1 protein expression by 71.1% (p< 0.05). CsA treatment significantly diminished the glomerular ZO-1 expression in the PAN rats as assessed by immunohistochemistry. CsA treatment significantly reduced proteinuria and the diminished glomerular ZO-1 expression in a PAN nephrosis rat model. These findings suggest the potential role of the slit diaphragm associated proteins in the development of the nephrotic syndrome, and CsA decreased the proteinuria probably by a direct action on the expression of these proteins in podocytes. Further investigations are needed to clarify the role of slit diaphragm associated proteins in the development of PAN nephrosis.
Keyword
MeSH Terms
-
Animals
Antimetabolites, Antineoplastic
Cyclosporine/*pharmacology
Immunosuppressive Agents/*pharmacology
Kidney Glomerulus/*drug effects/metabolism
Male
Membrane Proteins/*metabolism
Nephrosis/chemically induced/*drug therapy/*metabolism
Phosphoproteins/*metabolism
Puromycin Aminonucleoside
Rats
Rats, Sprague-Dawley
Research Support, Non-U.S. Gov't
Figure
Cited by 1 articles
-
Effects of Interleukin-13 and Montelukast on the Expression of Zonula Occludens-1 in Human Podocytes
Se Jin Park, Moin A. Saleem, Ja-Ae Nam, Tae-Sun Ha, Jae Il Shin
Yonsei Med J. 2015;56(2):426-432. doi: 10.3349/ymj.2015.56.2.426.
Reference
-
1. Caulfield JP, Farquhar MG. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J Cell Biol. 1974. 63:883–903.2. Farquhar MG, Vernier RL, Good RA. Studies on familial nephrosis. II. Glomerular changes observed with the electron microscope. Am J Pathol. 1957. 33:791–817.3. Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001. 108:1583–1587.4. Kanwar YS, Liu ZZ, Kashihara N, Wallner EI. Current status of the structural and functional basis of glomerular filtration and proteinuria. Semin Nephrol. 1991. 11:390–413.5. Farquhar MG. Editorial: The primary glomerular filtration barrier--basement membrane or epithelial slits? Kidney Int. 1975. 8:197–211.6. Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974. 60:423–433.7. Graham RC Jr, Karnovsky MJ. Glomerular permeability. Ultrastructural cytochemical studies using peroxidases as protein tracers. J Exp Med. 1966. 124:1123–1134.8. Daniels BS, Deen WM, Mayer G, Meyer T, Hostetter TH. Glomerular permeability barrier in the rat. Functional assessment by in vitro methods. J Clin Invest. 1993. 92:929–936.9. Orikasa M, Matsui K, Oite T, Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol. 1988. 141:807–814.10. Kawachi H, Kurihara H, Topham PS, Brown D, Shia MA, Orikasa M, et al. Slit diaphragm-reactive nephritogenic MAb 5-1-6 alters expression of ZO-1 in rat podocytes. Am J Physiol. 1997. 273(6 Pt 2):F984–F993.11. Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003. 83:253–307.12. Smithies O. Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci USA. 2003. 100:4108–4113.13. Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990. 111:1255–1263.14. Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998. 1:575–582.15. Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000. 11:1–8.16. Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, et al. FAT is a component of glomerular slit diaphragms. Kidney Int. 2001. 59:1003–1012.17. Macconi D, Ghilardi M, Bonassi ME, Mohamed EI, Abbate M, Colombi F, et al. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol. 2000. 11:477–489.18. Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol. 1992. 141:805–816.19. Lieberman KV, Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol. 1996. 7:56–63.20. Gregory MJ, Smoyer WE, Sedman A, Kershaw DB, Valentini RP, Johnson K, et al. Long-term cyclosporine therapy for pediatric nephrotic syndrome: a clinical and histologic analysis. J Am Soc Nephrol. 1996. 7:543–549.21. Meyrier A, Noel LH, Auriche P, Callard P. Long-term renal tolerance of cyclosporin A treatment in adult idiopathic nephrotic syndrome. Collaborative Group of the Societe de Nephrologie. Kidney Int. 1994. 45:1446–1456.22. Meyrier A. Antiproteinuric and immunological effects of cyclosporin A in the treatment of glomerular diseases. Nephrol Dial Transplant. 1992. 7:Suppl 1. 80–84.23. Jameson MD SV, Sharma R, Lovell HB, Diederich DA. Cyclosporine treatment decreases glomerular ultrafiltration coefficient. 1989. In : Proc Natl Kidney Found 19th Annu Sci Meet; 1989; Washington. –Abstract A12.24. Zietse R, Wenting GJ, Kramer P, Schalekamp MA, Weimar W. Effects of cyclosporin A on glomerular barrier function in the nephrotic syndrome. Clin Sci (Lond). 1992. 82:641–650.25. Kokui K, Yoshikawa N, Nakamura H, Itoh H. Cyclosporin reduces proteinuria in rats with aminonucleoside nephrosis. J Pathol. 1992. 166:297–301.26. Luimula P, Sandstrom N, Novikov D, Holthofer H. Podocyte-associated molecules in puromycin aminonucleoside nephrosis of the rat. Lab Invest. 2002. 82:713–718.27. Comper WD, Glasgow EF. Charge selectivity in kidney ultrafiltration. Kidney Int. 1995. 47:1242–1251.28. Goode NP, Shires M, Davison AM. The glomerular basement membrane charge-selectivity barrier: an oversimplified concept? Nephrol Dial Transplant. 1996. 11:1714–1716.29. Bridges CR, Myers BD, Brenner BM, Deen WM. Glomerular charge alterations in human minimal change nephropathy. Kidney Int. 1982. 22:677–684.30. Caulfield JP, Reid JJ, Farquhar MG. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976. 34:43–59.31. Diamond JR, Karnovsky MJ. Focal and segmental glomerulosclerosis following a single intravenous dose of puromycin aminonucleoside. Am J Pathol. 1986. 122:481–487.32. Venkatachalam MA, Cotran RS, Karnovsky MJ. An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J Exp Med. 1970. 132:1168–1180.33. Ryan GB, Karnovsky MJ. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975. 8:219–232.34. Luimula P, Ahola H, Wang SX, Solin ML, Aaltonen P, Tikkanen I, et al. Nephrin in experimental glomerular disease. Kidney Int. 2000. 58:1461–1468.35. Brodehl J, Brandis M, Helmchen U, Hoyer PF, Burghard R, Ehrich JH, et al. Cyclosporin A treatment in children with minimal change nephrotic syndrome and focal segmental glomerulosclerosis. Klin Wochenschr. 1988. 66:1126–1137.36. Meyrier A, Simon P, Perret G, Condamin-Meyrier MC. Remission of idiopathic nephrotic syndrome after treatment with cyclosporin A. Br Med J (Clin Res Ed). 1986. 292:789–792.37. Tejani AT, Butt K, Trachtman H, Suthanthiran M, Rosenthal CJ, Khawar MR. Cyclosporine A induced remission of relapsing nephrotic syndrome in children. Kidney Int. 1988. 33:729–734.38. Tejani A, Ingulli E. Cyclosporin in steroid-resistant idiopathic nephrotic syndrome. Contrib Nephrol. 1995. 114:73–77.39. Korbet SM. Treatment of primary focal segmental glomerulosclerosis. Kidney Int. 2002. 62:2301–2310.40. Bustos C, Gonzalez-Cuadrado S, Ruiz-Ortega M, Gomez-Guerrero C, Gonzalez E, Plaza JJ, et al. Cyclosporin A (CsA) modulates the glomerular production of inflammatory mediators and proteoglycans in experimental nephrosis. Clin Exp Immunol. 1995. 102:608–613.41. Wang JS, Yang AH, Chen SM, Young TK, Chiang H, Liu HC. Amelioration of antioxidant enzyme suppression and proteinuria in cyclosporin-treated puromycin nephrosis. Nephron. 1993. 65:418–425.42. Meyrier A. Treatment of glomerular disease with cyclosporin A. Nephrol Dial Transplant. 1989. 4:923–931.43. Chen D, Jefferson B, Harvey SJ, Zheng K, Gartley CJ, Jacobs RM, et al. Cyclosporine a slows the progressive renal disease of alport syndrome (X-linked hereditary nephritis): results from a canine model. J Am Soc Nephrol. 2003. 14:690–698.44. Berden JHM, Assmann KJM, Koene RAP. Antiproteinuric effect of cyclosporin (CsA) in anti-GBM nephritis in the mouse. J Autoimmun. 1992. 5:Suppl A. 185.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Puromycin Aminonucleoside on Podocyte P-Cadherin
- Effects of puromycin aminonucleoside on the cytoskeletal changes of glomerular epithelial cells
- Effect of Glomerular Cyclooxygenase-2 Overexpression on Podocyte Injury Induced by Puromycin in Mice
- The Change of Podocyte beta-Catenin by Puromycin Aminonucleoside
- Association of Proteinuria with Urinary Concentration Defect in Puromycin Aminonucleoside Nephrosis