Yonsei Med J.
2005 Feb;46(1):78-85. 10.3349/ymj.2005.46.1.78.
Serum Levels of Advanced Glycation End Products Are Associated with In-Stent Restenosis in Diabetic Patients
- Affiliations
-
- 1Division of Cardiology, Yonsei University College of Medicine, Seoul, Korea. kwonhm@yumc. yonsei.ac.kr
- 2Endocrinology and Metabolism, Yonsei University College of Medicine, Seoul, Korea.
- 3Hyonam Kidney Laboratory, Soonchunhyang University, Seoul, Korea.
- 4Division of Cardiology, Kwandong University College of Medicine, Koyang, Korea.
- KMID: 2158116
- DOI: http://doi.org/10.3349/ymj.2005.46.1.78
Abstract
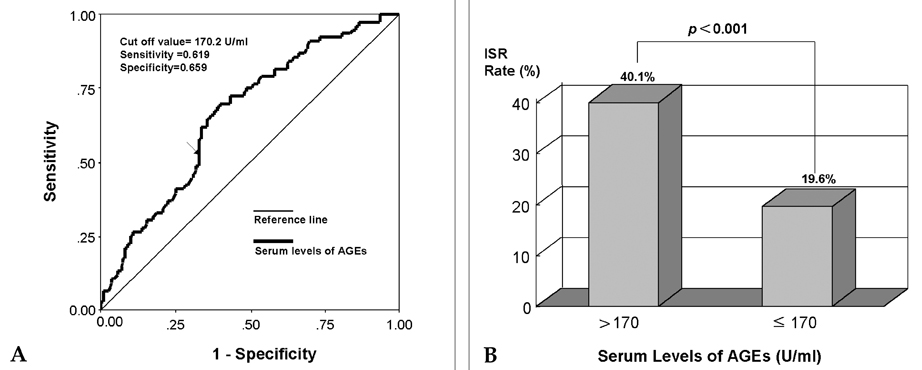
- The formation of advanced glycation end products (AGEs), in various tissues has been known to enhance immunoinflammatory reactions and local oxidant stresses in long standing diabetes. Recently, AGEs have been reported to play a role in neointimal formation in animal models of arterial injury. We attempted to determine whether the serum levels of AGEs are associated with coronary restenosis in diabetic patients. Blood samples were collected from diabetic patients with coronary artery disease undergoing stent implantation and the serum levels of AGEs were analyzed by the fluorescent intensity method. The development of in-stent restenosis (ISR) was evaluated by a 6-month follow-up coronary angiography. A total of 263 target lesions were evaluated, in 203 patients. The ISR rate in the high-AGE (> 170 U/ml) group (40.1%) was significantly higher than in the low-AGE group (< or =170 U/ml) (19.6%) (p < 0.001). Furthermore, multivariate analysis revealed that a high level of serum AGEs is an independent risk factor for the development of ISR (odds ratio, 2.659; 95% CI, 1.431-4.940; p=0.002). The serum levels of AGEs constitute an excellent predictive factor for ISR, and should be one of the guidelines for medical therapy and interventional strategy to prevent ISR in diabetic patients.
MeSH Terms
Figure
Reference
-
1. Belle EV, Bauters C, Hubert E, Bodart JC, Abolmaali K, Meurice T, et al. Restenosis rates in diabetic patients: a comparison of coronary stenting and balloon angioplasty in native coronary arteries. Circulation. 1997. 96:1454–1460.2. Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998. 31:224–230.3. Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003. 93:1159–1169.4. Zhou Z, Wang K, Penn MS, Marso SP, Lauer MA, Forudi F, et al. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003. 107:2238–2243.5. Stephenson K, Tunstead J, Tsai A, Gordon R, Henderson S, Dansky HM. Neointimal formation after endovascular arterial injury is markedly attenuated in db/db mice. Arterioscler Thromb Vasc Biol. 2003. 23:2027–2033.6. Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003. 111:959–972.7. Tanaka S, Avigad G, Brodsky B, Eikenberry EF. Glycation induces expansion of the molecular packing of collagen. J Mol Biol. 1988. 203:495–505.8. Haitoglou CS, Tsilbary EC, Brownlee M, Charonis AS. Altered cellular interactions between endothelial cells and nonenzymatically glycosylated laminin/type IV collagen. J Biol Chem. 1992. 267:12404–12407.9. Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul V, Topol EJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty multivessel coronary disease: implications for patient selection. Circulation. 1990. 82:1193–1202.10. Wrobel K, Wrobel K, Garay-Sevilla ME, Nava LE, Malacara JM. Novel analytical approach to monitoring advanced glycosylation end products in human serum with on-line spectrophotometric and spectrofluorometric detection in a flow system. Clin Chem. 1997. 43:1563–1569.11. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979. 241:2035–2038.12. Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Diabetes control and complications trial; Epidemiology of diabetes interventions and complications research group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes. N Engl J Med. 2003. 348:2294–2303.13. West NEJ, Ruygrok PN, Disco CMC, Webster MWI, Lindeboom WK, O'Neill WW, et al. Clinical and angiographic predictors of restenosis after stent deployment in diabetic patients. Circulation. 2004. 109:867–873.14. Brownlee M, Cerami A, Vlassara H. Advanced glycation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988. 318:1315–1321.15. Niwa T. 3-Deoxyglucosone metabolism, analysis, biological activity, and clinical implication. J Chromatogr B Biomed Sci Appl. 1999. 731:23–36.16. Hasuike Y, Nakanishi T, Otaki Y, Nanami M, Tanimoto T, Taniguchi N, et al. Plasma 3-deoxyglucosone elevation in chronic renal failure is associated with increased aldose reductase in erythrocytes. Am J Kidney Dis. 2002. 40:464–471.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Advanced Glycation End Products in Diabetic Vascular Complications
- Inhibition of advanced glycation end product formation by burdock root extract
- Vascular Endothelial Growth Factor (VEGF) and Advanced Glycation End Products (AGEs) Overexpression in the Retina and Serum and Lens Opacities of Streptozotocin-induced Diabetic Rats
- Advanced Glycation End Products and Diabetic Complications
- Letter: The Association between Serum Endogenous Secretory Receptor for Advanced Glycation End Products and Vertebral Fractures in Type 2 Diabetes (Endocrinol Metab 2012;27:289-94, Cheol Ho Lee et al.)