Yonsei Med J.
2005 Feb;46(1):73-77. 10.3349/ymj.2005.46.1.73.
Serial Assessment of Myocardial Properties Using Cyclic Variation of Integrated Backscatter in an Adriamycin- Induced Cardiomyopathy Rat Model
- Affiliations
-
- 1Cardiology Division, Yonsei University College of Medicine, Seoul, Korea. jwha@yumc.yonsei.ac.kr
- 2Department of Nuclear Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2158115
- DOI: http://doi.org/10.3349/ymj.2005.46.1.73
Abstract
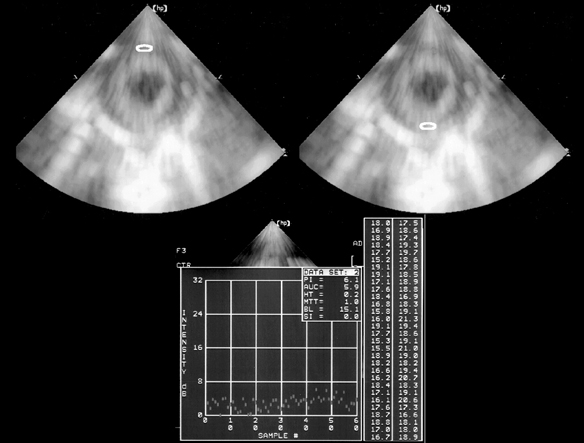
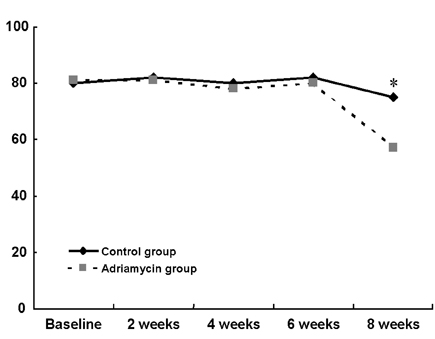
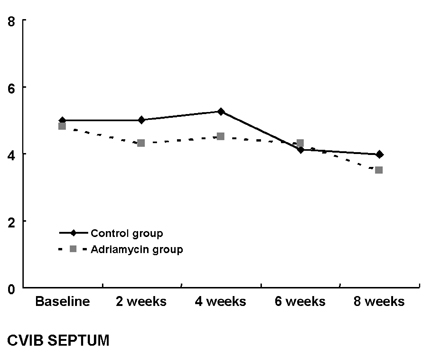
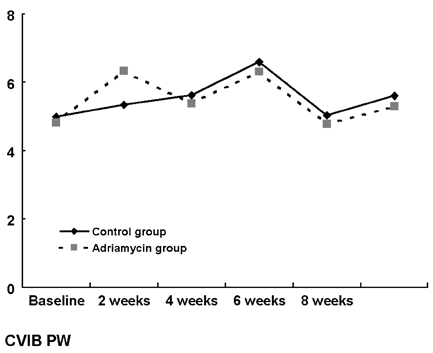
- Although adriamycin (Doxorubicin) is one of the most effective and useful antineoplastic agents for the treatment of a variety of malignancies, its repeated administration can induce irreversible myocardial damage and resultant heart failure. Currently, no marker to detect early cardiac damage is available. The purpose of this study was to investigate whether an assessment of the acoustic properties of the myocardium could enable the earlier detection of myocardial damage after adriamycin chemotherapy. Forty Wistar rats were treated with adriamycin (2 mg/kg, i.v.) once a week for 2, 4, 6 or 8 weeks consecutively. Left ventricular ejection fraction (LVEF) was calculated using M-mode echocardiography data. The magnitude of cardiac cycle dependent variation of integrated backscatter (CVIB) of the myocardium was measured in the mid segment of the septum and in the posterior wall of the left ventricle, using a real time two dimensional integrated backscatter imaging system. LVEF was significantly lower in the adriamycin-treated 8-week group than in the controls (75 +/- 9 vs 57 +/- 8%, p < 0.05). Myocyte damage was only seen in the 8-week adriamycin-treated group. However, no significant changes of CVIB were observed between baseline or during follow-up in the ADR or control group. In conclusion, serial assessment of the acoustic properties of the myocardium may not be an optimal tool for the early detection of myocardial damage after doxorubicin chemotherapy in a rat model.
Keyword
MeSH Terms
Figure
Reference
-
1. Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996. 125:47–58.2. Jeon TJ, Lee JD, Ha JW, Yang WI, Cho SH. Evaluation of cardiac adrenergic neuronal damage in rats with doxorubicin-induced cardiomyopathy using iodine-131 MIBG autoradiography and PGP 9.5 immunohistochemistry. Eur J Nucl Med. 2000. 27:686–693.3. Vered Z, Mohr GA, Barzilai B, Gessler CJ Jr, Wickline SA, Wear KA, et al. Ultrasound integrated backscatter tissue characterization of remote myocardial infarction in human subjects. J Am Coll Cardiol. 1989. 13:84–91.4. Pasquet A, D'Hondt AM, Melin JA, Vanoverschelde JL. Relation of ultrasonic tissue characterization with integrated backscatter to contractile reserve in chronic left ventricular ischemic dysfunction. Am J Cardiol. 1998. 81:68–74.5. Takiuchi S, Ito H, Iwakura K, Taniyama Y, Nishikawa N, Masuyama T, et al. Ultrasonic tissue characterization predicts myocardial viability in early stage of reperfused acute myocardial infarction. Circulation. 1998. 97:356–362.6. Vitale DF, Bonow RO, Gerundo G, Pelaggi N, Lauria G, Leosco D, et al. Alterations in ultrasonic backscatter during exercise-induced myocardial ischemia in humans. Circulation. 1995. 92:1452–1457.7. Colonna P, Montisci R, Galiuto L, Meloni L, Iliceto S. Effects of acute myocardial ischemia on intramyocardial contraction heterogeneity. A study performed with ultrasound integrated backscatter during transesophageal atrial pacing. Circulation. 1999. 100:1770–1776.8. Iliceto S, Galiuto L, Colonna P, Napoli VF, Rizzon P. Effects of atrial pacing stress test on ultrasonic integrated backscatter cyclic variations in normals and in patients with coronary artery disease. Eur Heart J. 1997. 18:1590–1598.9. Masuyama T, St Goar FG, Tye TL, Oppenheim G, Schnittger I, Popp RL. Ultrasonic tissue characterization of human hypertrophied hearts in vivo with cardiac cycle-dependent variation in integrated backscatter. Circulation. 1989. 80:925–934.10. Vered Z, Barzilai B, Mohr GA, Thomas LJ 3rd, Genton R, Sobel BE, et al. Quantitative ultrasonic tissue characterization with real-time integrated backscatter imaging in normal human subjects and in patients with dilated cardiomyopathy. Circulation. 1987. 76:1067–1073.11. Masuyama T, Valantine HA, Gibbons R, Schnittger I, Popp RL. Serial measurement of integrated ultrasonic backscatter in human cardiac allografts for the recognition of acute rejection. Circulation. 1990. 81:829–839.12. Perez JE, McGill JB, Santiago JV, Schechtman KB, Waggoner AD, Miller JG, et al. Abnormal myocardial acoustic properties in diabetic patients and their correlation with the severity of disease. J Am Coll Cardiol. 1992. 19:1154–1162.13. Naito J, Masuyama T, Mano T, Kondo H, Doi Y, Yamamoto K, et al. Dobutamine stress ultrasonic myocardial tissue characterization in patients with dilated cardiomyopathy. J Am Soc Echocardiogr. 1996. 9:470–479.14. Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL Jr, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981. 64:744–753.15. Lattanzi F, Spirito P, Picano E, Mazzarisi A, Landini L, Distante A, et al. Quantitative assessment of ultrasonic myocardial reflectivity in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1991. 17:1085–1090.16. Naito J, Masuyama T, Tanouchi J, Mano T, Kondo H, Yamamoto K, et al. Analysis of transmural trend of myocardial integrated ultrasound backscatter for differentiation of hypertrophic cardiomyopathy and ventricular hypertrophy due to hypertension. J Am Coll Cardiol. 1994. 24:517–524.17. Angermann CE, Nassau K, Stempfle HU, Kruger TM, Drewello R, Junge R, et al. Recognition of acute cardiac allograft rejection from serial integrated backscatter analyses in human orthotopic heart transplant recipients: comparison with conventional echocardiography. Circulation. 1997. 95:140–150.18. Di Bello V, Talarico L, Picano E, Di Muro C, Landini L, Paterni M, et al. Increased echodensity of myocardial wall in the diabetic heart: an ultrasound tissue characterization study. J Am Coll Cardiol. 1995. 25:1408–1415.19. Nagai H, Omi W, Yuasa T, Sakagami S, Takata S, Kobayashi K. Ultrasonic analysis of anthracycline-induced myocardial damage using cyclic variation of integrated backscatter. J Am Soc Echocardiogr. 2003. 16:808–813.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Preventive Effect of Dexrazoxane and Pentoxifylline on Adriamycin Induced Cardiomyopathy
- Serum Lipid Levels and Fatty Acid Metabolism in the Rat With Adriamycin Induced Cardiomyopathy
- Radiologic evaluation of adriamycin induced toxic cardiomyopathy in childhood leukemia
- Losartan Reduces Remodeling and Apoptosis in an Adriamycin-Induced Cardiomyopathy Rat Model
- The Role of Apoptosis in Adriamycin Induced Cardiotoxicity and Preventive Effect of L-carnitine in Rat