Yonsei Med J.
2005 Feb;46(1):34-41. 10.3349/ymj.2005.46.1.34.
Effects of Methylphenidate on Quantitative EEG of Boys with Attention-deficit Hyperactivity Disorder in Continuous Performance Test
- Affiliations
-
- 1Department of Psychiatry, Yonsei University College of Medicine, Seoul, Korea. dhsong@yumc.yonsei.ac.kr
- 2Department of Psychiatry, Seongkyunkwan University, Seoul, Korea.
- 3Department of Neuropsychiatry, National Health Insurance Corporation Ilsan Hospital, Kyunggi-do, Korea.
- 4Department of Child Welfare, Sookmyung University, Seoul, Korea.
- KMID: 2158110
- DOI: http://doi.org/10.3349/ymj.2005.46.1.34
Abstract
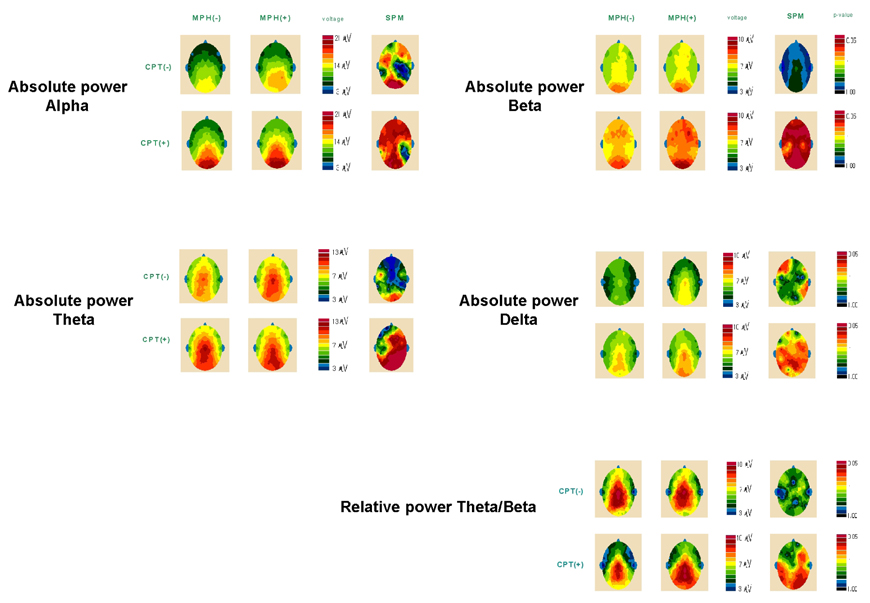
- The purpose of this study was to investigate the effects of methylphenidate, a psychostimulant, on quantitative electroencephalography (QEEG) during the continuous performance test (CPT) in boys with attention-deficit hyperactivity disorder (ADHD). The QEEG was obtained from 20 boys with ADHD. The amplitudes of 4 bands (alpha, beta, delta, and theta) in the QEEG, as well as the theta/beta ratio, before and after the administration of methylphenidate were compared during both the resting and CPT states. Methylphenidate induced a significant increase of alpha activities in both the right and left frontal and occipital areas, an increase of beta activities in almost all areas except for the temporal region, a decrease of theta activities in both the occipital and right temporo-parietal areas, a mild decrease of delta activities in the occipito-parietal areas, and an increase of the theta/beta ratio in the right frontal and parieto-occipital, and left temporal areas during the CPT state. No significant QEEG changes were induced by the administration of methylphenidate in the resting state. These data suggest that methylphenidate has greater electrophysiological influences on the cerebral topographical activities during the performance of attentional tasks, as compared to the resting state, in boys with ADHD.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Effect of Hippotherapy and Medication on Children with Attention Deficit Hyperactivity Disorder: A Pilot Study
Jihye Song, Byongsu Jang, Jiwon Kim, Jiyoung Lee, Hye-Yeon Shin, Yunhye Oh, Kounseok Lee, Seonwoo Kim, Yun-Hee Kim, Jeong-Yi Kwon, Yoo-Sook Joung
J Korean Neuropsychiatr Assoc. 2015;54(1):112-118. doi: 10.4306/jknpa.2015.54.1.112.
Reference
-
1. Rapport MD, Kelly KL. Psychostimulant effects on learning and cognitive function: Findings and implication for children with attention deficit hyperactivity disorder. Clin Psychol Rev. 1991. 11:61–92.2. Coon HW, Klorman R, Borgstedt AD. Effects of methylphenidate on adolescents with a childhood history of attention deficit disorder: II. Information processing. J Am Acad Child Adolesc Psychiatry. 1987. 26:368–374.3. Garfinkel BD, Brown WA, Klee SH, Braden W, Beauchesne H, Shapiro SK. Neuroendocrine and cognitive responses to amphetamine in adolescents with a history of attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1986. 25:503–508.4. Jonh ER, Prichep LS, Ahn H, Easton P, Fridman J, Kaye H. Neurometric evaluation of cognitive dysfunctions and neurological disorders in children. Prog Neurobiol. 1983. 21:239–290.5. Prichep LS, John ER. QEEG profiles of psychiatric disorders. Brain Topogr. 1992. 4:249–257.6. John ER, Prichep LS, Almas M. Subtyping of psychiatric patients by cluster analysis of QEEG. Brain Topogr. 1992. 4:321–326.7. Callaway E, Halliday R, Naylor H. Hyperactive children's event-related potentials fail to support underarousal and maturational-lag theories. Arch Gen Psychiatry. 1983. 40:1243–1248.8. Kuperman S, Johnson B, Arndt S, Lindgren S, Wolraich M. Quantitative EEG differences in a nonclinical sample of children with ADHD and undifferentiated ADD. J Am Acad Child Adolesc Psychiatry. 1996. 38:1009–1017.9. Mann CA, Lubar J, Zimmerman A, Miller C, Muenchen R. Quantitative analysis of EEG in boys with attention deficit hyperactivity disorder: Controlled study with clinical implications. Pediatr Neurol. 1992. 8:30–36.10. Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol Psychiatry. 1996. 40:951–963.11. Lubar JF, Swartwood MO, Swartwood JN, Timmermann DL. Quantitative EEG and auditory ERP in the evaluation of attention-deficit/hyperactivity disorder: Effects of methylphenidate and implications for neurofeedback training. J Psychoeduc Assess. 1995. 34:143–160.12. Lubar JF, White JN, Swartwood MO, Swartwood JN. Methylphenidate effects on global and complex measures of EEG. Pediatr Neurol. 1999. 21:633–637.13. Loo SK, Specter E, Smolen A, Hopfer C, Teale PD, Reite MI. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. J Am Acad Child Adolesc Psychiatry. 2003. 42:986–993.14. Verbaten MN, Overtoom CCE, Koelega HS, Swaab-Barneveld H, Gaag RJ, Buitelaar J, et al. Methylphenidate influences on both early and late ERP waves of ADHD children in a continuous performance test. J Abnorm Child Psychol. 1994. 22:561–578.15. Winsberg BG, Javitt DC, Shanahan/Silipo G. Electrophysiological indices of information processing in methylphenidate responders. Biol Psychiatry. 1997. 42:434–445.16. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2000. 4th ed. Washington DC: American Psychiatric Association Press.17. Conners CK. The Conners rating scale: Instruments for the assessment of childhood psychopathology. 1985. Washington, D.C.: Children's Hospital National Medical Center;Unpublished manuscript.18. Greenberg LM, Waldman ID. Developmental normative data on the Test of Variables of Attention (TOVA). J Child Psychol Psychiatry. 1993. 34:1019–1030.19. Ahn CB, Park DJ, Yoo SK, Lee SH, Ham YJ. 32-channel EEG and evoked potential mapping system. J Med Eng Technol. 1996. 17:179–189.20. Spencer T, Biederman J, Wilens T, Greene R. Martin A, Scahill L, Charney DS, Leckman JF, editors. Attention Deficit Hyperactivity Disorder. Pediatric psychopharmacology. 2003. New York: Oxford;447–465.21. Halperin JM, Newcorn JH, Sharma V. Greenhill LL, Osman BB, editors. Ritalin: Diagnostic comorbidity and attentional measures. Ritalin: Theory and patient management. 1991. New York: Mary Ann Liebert;15–24.22. Corkum PV, Siegel LS. Is the continuous performance task a valuable research tool for use with children with attention-deficit-hyperactivity disorder? J Child Psychol Psychiatry. 1993. 34:1217–1239.23. Satterfield JH, Schell AM, Nicholas T, Backs RW. Topographic study of auditory event-related potentials in normal boys and boys with attention deficit disorder with hyperactivity. Psychophysiology. 1988. 25:591–606.24. Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD. Methylphenidate speeds evaluation processes of attention deficit disorder adolescents during a continuous performance test. J Abnorm Child Psychol. 1991. 19:263–283.25. Young ES, Perros P, Price GW, Sadler T. Acute challenge ERP as a prognostic of stimulant therapy outcome in attention-deficit hyperactivity disorder. Biol Psychiatry. 1995. 37:25–33.26. Voeller KK. What can neurological models of attention, intention, and arousal tell us about attention deficit hyperactive disorder? J Neuropsychiatry Clin Neurosci. 1991. 3:209–216.27. Carter CS, Krener P, Chaderjian M, Northcutt C, Wolfe V. Asymmetrical visual-spatial attentional pdrformance in ADHD: Evidence for a right hemispheric deficit. Biol Psychiatry. 1995. 37:789–797.28. Heilman KM, Voeller KK, Nadeau SE. A possible pathophysiological substrate of attention deficit hyperactivity disorder. J Child Neurol. 1991. 6:suppl. 76–81.29. Mitchell WG, Chavez JM, Baker SD, Guzman BL, Azen SP. Reaction time, impulsivity and attention in hyperactive children and controls: A video game technique. J Child Neurol. 1990. 5:195–204.30. Ucles P, Lorente S. Electrophysiologic measures of delayed maturation in attention-deficit hyperactivity disorder. J Child Neurol. 1996. 11:155–156.31. Matochik JA, Liebenauer LL, King AC, Szymanski HV, Cohen RM, Zametkin AJ. Cerebral glucose metabolism in adults with attention deficit hyperactivity disorder after chronic stimulant treatment. Am J Psychiatry. 1994. 151:658–664.32. Barkley RA. ADHD and the nature of self-control. 1997. New York: Guilford Press.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- OROS Methylphenidate Treatment of Secondary Adult ADHD after Traumatic Brain Injury
- The Effectiveness of Methylphenidate in the Treatment of Encopresis Independent from Attention-Deficit Hyperactivity Disorder Symptoms
- Cerebral Functional Localization Related to Attentional Work in Patients with Attention-Deficit Hyperactivity Disorder
- Quantitative Morphologic Analysis Using Magnetic Resonance Imaging of the Corpus Callosum and Lateral Ventricle in Boys with Attention Deficit Hyperactivity Disorder
- Pharmacological treatment for attention deficit hyperactivity disorder in adults