J Korean Med Sci.
2012 Apr;27(4):395-401. 10.3346/jkms.2012.27.4.395.
Effects of Postnatal Dexamethasone or Hydrocortisone in a Rat Model of Antenatal Lipopolysaccharide and Neonatal Hyperoxia Exposure
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea. beyil@snu.ac.kr
- 2Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Pediatrics, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2157900
- DOI: http://doi.org/10.3346/jkms.2012.27.4.395
Abstract
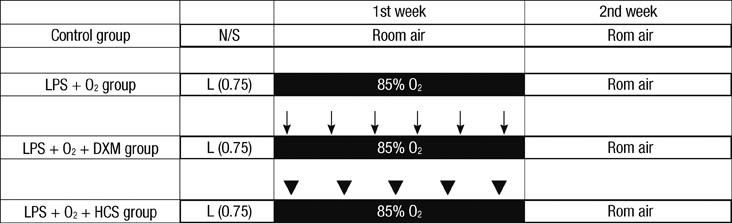
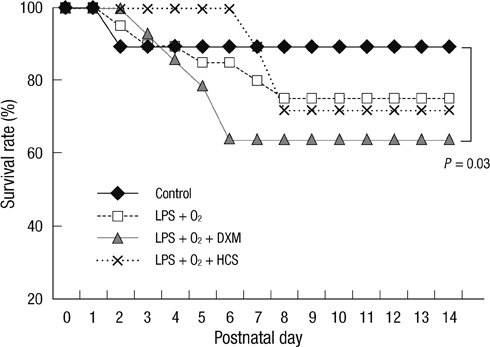
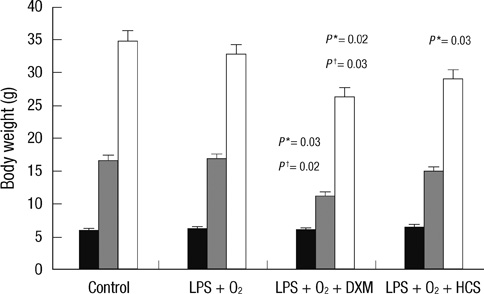
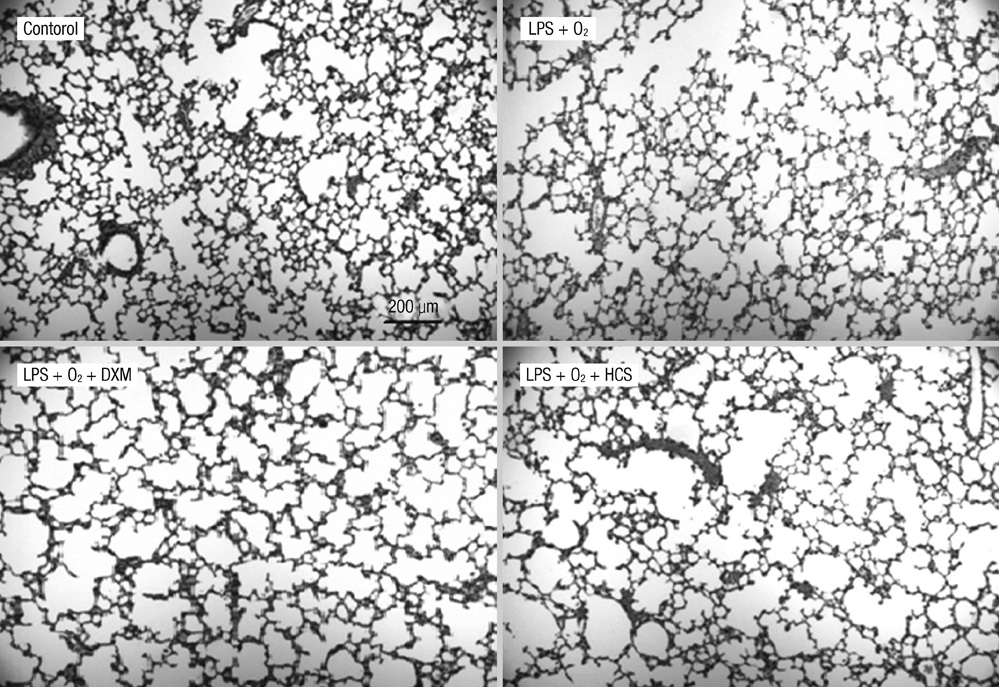
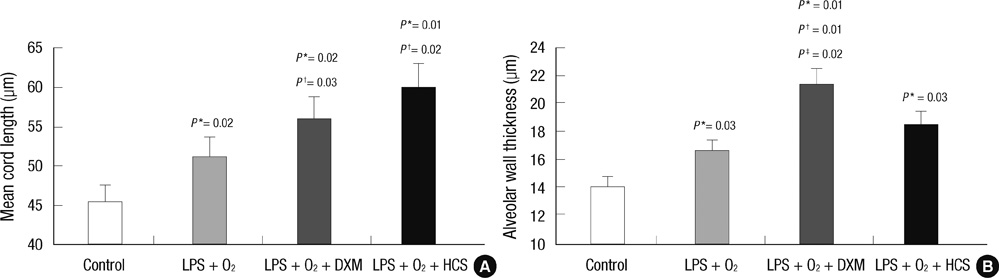
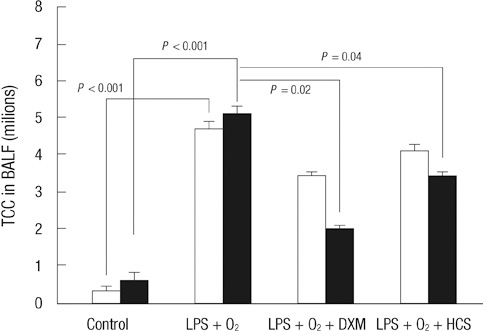
- The aim of our study was to investigate the differential effects of dexamethasone (DXM) and hydrocortisone (HCS) on somatic growth and postnatal lung development in a rat model of bronchopulmonary dysplasia (BPD). A rat model of BPD was induced by administering intra-amniotic lipopolysaccharide (LPS) and postnatal hyperoxia. The rats were treated with a 6-day (D1-D6) tapering course of DXM (starting dose 0.5 mg/kg/day), HCS (starting dose 2 mg/kg/day), or an equivalent volume of normal saline. DXM treatment in a rat model of BPD induced by LPS and hyperoxia was also associated with a more profound weight loss compared to control and LPS + O2 groups not exposed to corticosteroid, whereas HCS treatment affected body weight only slightly. Examination of lung morphology showed worse mean cord length in both LPS + O2 + DXM and LPS + O2 + HCS groups as compared to the LPS + O2 alone group, and the LPS + O2 + DXM group had thicker alveolar walls than the LPS + O2 group at day 14. The HCS treatment was not significantly associated with aberrant alveolar wall thickening and retarded somatic growth. The use of postnatal DXM or HCS in a rat model of BPD induced by intra-amniotic LPS and postnatal hyperoxia appeared detrimental to lung growth, but there was less effect in the case of HCS. These findings suggest that effect of HCS on somatic growth and pulmonary outcome may be better tolerated in neonates for preventing and/or treating BPD.
MeSH Terms
-
Amnion/drug effects
Animals
Animals, Newborn
Anti-Inflammatory Agents/*pharmacology
Dexamethasone/*pharmacology
Disease Models, Animal
Female
Hydrocortisone/*pharmacology
*Hyperoxia
Lipopolysaccharides/toxicity
Lung Diseases/*pathology
Oxygen/metabolism
Pulmonary Alveoli/*drug effects/growth & development/pathology
Rats
Rats, Sprague-Dawley
Figure
Cited by 1 articles
-
Rosiglitazone, a Peroxisome Proliferator-Activated Receptor-γ Agonist, Restores Alveolar and Pulmonary Vascular Development in a Rat Model of Bronchopulmonary Dysplasia
Hyun Ju Lee, Youn Jin Lee, Chang Won Choi, Jin-A Lee, Ee-Kyung Kim, Han-Suk Kim, Beyong Il Kim, Jung-Hwan Choi
Yonsei Med J. 2014;55(1):99-106. doi: 10.3349/ymj.2014.55.1.99.
Reference
-
1. Yoder MC Jr, Chua R, Tepper R. Effect of dexamethasone on pulmonary inflammation and pulmonary function of ventilator-dependent infants with bronchopulmonary dysplasia. Am Rev Respir Dis. 1991. 143:1044–1048.2. Halliday HL. Clinical trials of postnatal corticosteroids: inhaled and systemic. Biol Neonate. 1999. 76:29–40.3. Halliday HL, Ehrenkranz RA, Doyle LW. Early postnatal (< 96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2003. CD001146.4. Halliday HL, Ehrenkranz RA. Moderately early (7-14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2001. CD001144.5. Doyle LW, Ehrenkranz RA, Halliday HL. Postnatal hydrocortisone for preventing or treating bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010. 98:111–117.6. Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, Abbasi S, Cole CH, Aucott SW, Thilo EH, Rozycki HJ, Lacy CB. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics. 2007. 120:40–48.7. Watterberg KL, Gerdes JS, Gifford KL, Lin HM. Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics. 1999. 104:1258–1263.8. Thébaud B, Lacaze-Masmonteil T, Watterberg K. Postnatal glucocorticoids in very preterm infants: "the good, the bad, and the ugly"? Pediatrics. 2001. 107:413–415.9. Choi CW, Kim BI, Hong JS, Kim EK, Kim HS, Choi JH. Bronchopulmonary dysplasia in a rat model induced by intra-amniotic inflammation and postnatal hyperoxia: morphometric aspects. Pediatr Res. 2009. 65:323–327.10. Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963. 12:131–155.11. Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001. 32:76–91.12. Gross I. Regulation of fetal lung maturation. Am J Physiol. 1990. 259:L337–L344.13. Vyas J, Kotecha S. Effects of antenatal and postnatal corticosteroids on the preterm lung. Arch Dis Child Fetal Neonatal Ed. 1997. 77:F147–F150.14. Massaro GD, Massaro D. Formation of alveoli in rats: postnatal effect of prenatal dexamethasone. Am J Physiol. 1992. 263:L37–L41.15. Tschanz SA, Makanya AN, Haenni B, Burri PH. Effects of neonatal high-dose short-term glucocorticoid treatment on the lung: a morphologic and morphometric study in the rat. Pediatr Res. 2003. 53:72–80.16. Tschanz SA, Burri PH. Postnatal lung development and its impairment by glucocorticoids. Pediatr Pulmonol Suppl. 1997. 16:247–249.17. Tschanz SA, Damke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. Biol Neonate. 1995. 68:229–245.18. Sahebjami H, Domino M. Effects of postnatal dexamethasone treatment on development of alveoli in adult rats. Exp Lung Res. 1989. 15:961–973.19. Fayon M, Jouvencel P, Carles D, Choukroun ML, Marthan R. Differential effect of dexamethasone and hydrocortisone on alveolar growth in rat pups. Pediatr Pulmonol. 2002. 33:443–448.20. Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, Couser RJ, Garland JS, Rozycki HJ, Leach CL, Backstrom C, Shaffer ML. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. 2004. 114:1649–1657.21. Rademaker KJ, Uiterwaal CS, Groenendaal F, Venema MM, van Bel F, Beek FJ, van Haastert IC, Grobbee DE, de Vries LS. Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm-born children. J Pediatr. 2007. 150:351–357.22. Leitch CA, Ahlrichs J, Karn C, Denne SC. Energy expenditure and energy intake during dexamethasone therapy for chronic lung disease. Pediatr Res. 1999. 46:109–113.23. Ohtsu N, Ariagno RL, Sweeney TE, Davis L, Moses L, Petriceks S, Daehne I, Bensch K, Northway WH Jr. The effect of dexamethasone on chronic pulmonary oxygen toxicity in infant mice. Pediatr Res. 1989. 25:353–359.24. Dallas DV, Keeney SE, Mathews MJ, Schmalstieg FC. Effects of postnatal dexamethasone on oxygen toxicity in neonatal rats. Biol Neonate. 2004. 86:145–154.25. Ohashi T, Takada S, Motoike T, Tsuneishi S, Matsuo M, Sano K, Nakamura H. Effect of dexamethasone on pulmonary surfactant metabolism in hyperoxia-treated rat lungs. Pediatr Res. 1991. 29:173–177.26. Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, Groenendaal F, de Vries LS, Huppi PS. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005. 116:1–7.27. Ozdemir A, Brown MA, Morgan WJ. Markers and mediators of inflammation in neonatal lung disease. Pediatr Pulmonol. 1997. 23:292–306.28. Deng H, Mason SN, Auten RL Jr. Lung inflammation in hyperoxia can be prevented by antichemokine treatment in newborn rats. Am J Respir Crit Care Med. 2000. 162:2316–2323.29. Groneck P, Reuss D, Gotze-Speer B, Speer CP. Effects of dexamethasone on chemotactic activity and inflammatory mediators in tracheobronchial aspirates of preterm infants at risk for chronic lung disease. J Pediatr. 1993. 122:938–944.30. Dik WA, Versnel MA, Naber BA, Janssen DJ, van Kaam AH, Zimmermann LJ. Dexamethasone treatment does not inhibit fibroproliferation in chronic lung disease of prematurity. Eur Respir J. 2003. 21:842–847.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Steroid use in Perinatal Medicine
- The Effect of Prenatal Dexamethasone Treatment on Pulmonary Antioxidant System in Newborn Rats during Prolonged High O2 Exposure
- Morphologic Changes of Lung Parenchymal Tissue in Neonatal Rat Pups Under Chronic Hyperoxia
- Evidence for adverse effect of perinatal glucocorticoid use on the developing brain
- Expression of Peroxiredoxin I and II in Neonatal and Adult Rat Lung Exposed to Hyperoxia