J Korean Med Sci.
2010 Oct;25(10):1499-1505. 10.3346/jkms.2010.25.10.1499.
Functional and Histologic Changes After Repeated Transcranial Direct Current Stimulation in Rat Stroke Model
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University, College of Medicine, Seoul, Korea. guitar1@snu.ac.kr
- 2Department of Pathology, Seoul National University, College of Medicine, Seoul, Korea.
- 3Department of Rehabilitation Medicine, Seoul St. Mary's Hospital, Seoul, Korea.
- 4Department of Neurology, Seoul National University, College of Medicine, Seoul, Korea.
- KMID: 2157856
- DOI: http://doi.org/10.3346/jkms.2010.25.10.1499
Abstract
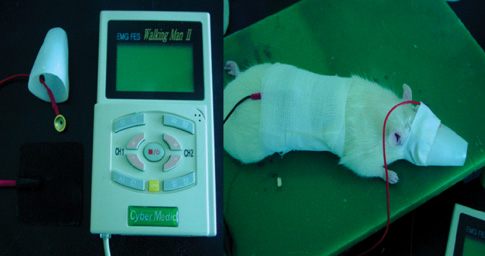
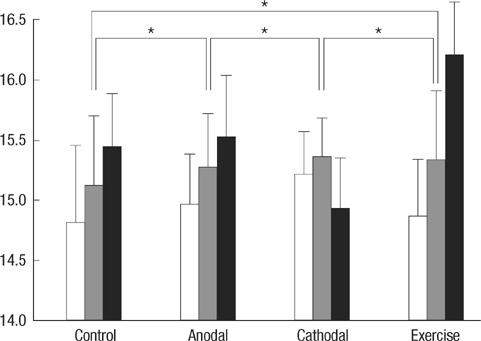
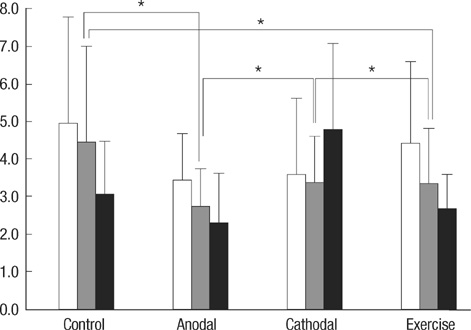
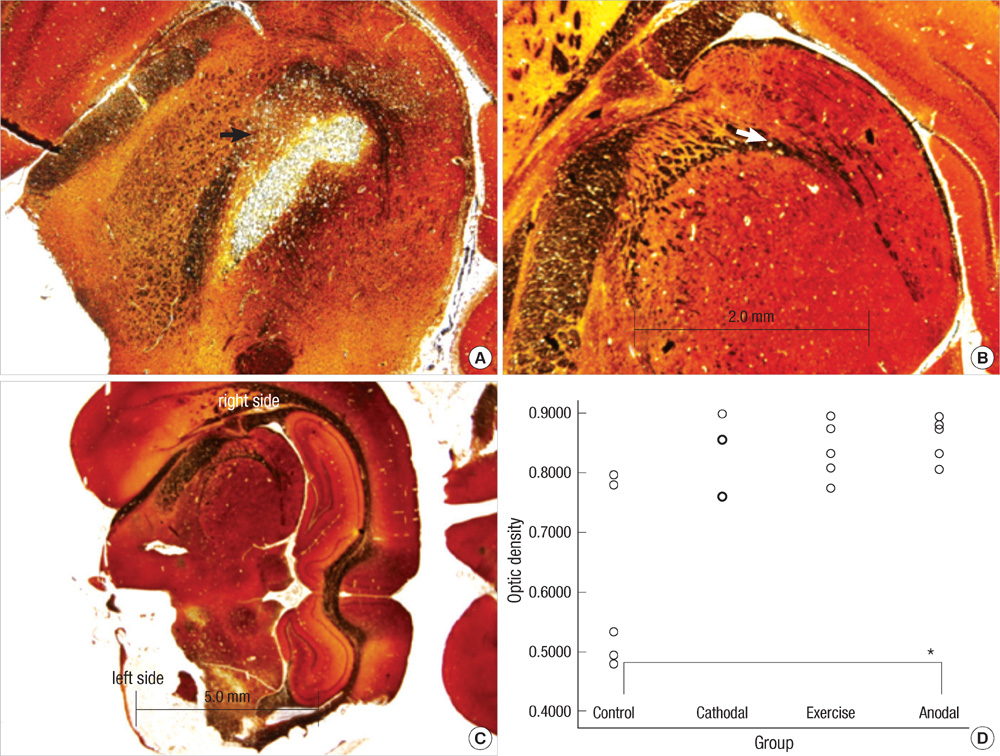
- Transcranial direct current stimulation (tDCS) is associated with enhancement or weakening of the NMDA receptor activity and change of the cortical blood flow. Therefore, repeated tDCS of the brain with cerebrovascular injury will induce the functional and histologic changes. Sixty-one Sprague-Dawley rats with cerebrovascular injury were used. Twenty rats died during the experimental course. The 41 rats that survived were allocated to the exercise group, the anodal stimulation group, the cathodal stimulation group, or the control group according to the initial motor function. Two-week treatment schedules started from 2 days postoperatively. Garcia, modified foot fault, and rota-rod performance scores were checked at 2, 9, and 16 days postoperatively. After the experiments, rats were sacrificed for the evaluation of histologic changes (changes of the white matter axon and infarct volume). The anodal stimulation and exercise groups showed improvement of Garcia's and modified foot fault scores at 16 days postoperatively. No significant change of the infarct volume happened after exercise and tDCS. Neuronal axons at the internal capsule of infarct hemispheres showed better preserved axons in the anodal stimulation group. From these results, repeated tDCS might have a neuroprotective effect on neuronal axons in rat stroke model.
MeSH Terms
Figure
Cited by 2 articles
-
Plasticity Associated Changes in Neurophysiological Tests Following Non Invasive Brain Stimulation in Stroke Rat Model
Min Kyun Sohn, Hee-Jung Song, Sungju Jee
Korean J Clin Neurophysiol. 2014;16(2):62-69. doi: 10.14253/kjcn.2014.16.2.62.Effects of Electric Cortical Stimulation (ECS) and Transcranial Direct Current Stimulation (tDCS) on Rats With a Traumatic Brain Injury
Ki Pi Yu, Yong-Soon Yoon, Jin Gyeong Lee, Ji Sun Oh, Jeong-Seog Lee, Taeyong Seog, Han-Young Lee
Ann Rehabil Med. 2018;42(4):502-513. doi: 10.5535/arm.2018.42.4.502.
Reference
-
1. Ding DC, Shyu WC, Lin SZ, Li H. Current concepts in adult stem cell therapy for stroke. Curr Med Chem. 2006. 13:3565–3574.
Article2. Liu DD, Shyu WC, Lin SZ. Stem cell therapy in stroke: strategies in basic study and clinical application. Acta Neurochir Suppl. 2006. 99:137–139.
Article3. Boggio PS, Alonso-Alonso M, Mansur CG, Rigonatti SP, Schlaug G, Pascual-Leone A, Fregni F. Hand function improvement with low-frequency repetitive transcranial magnetic stimulation of the unaffected hemisphere in a severe case of stroke. Am J Phys Med Rehabil. 2006. 85:927–930.
Article4. Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005. 16:1551–1555.
Article5. Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006. 8:203–204.
Article6. Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke. Lancet Neurol. 2006. 5:708–712.
Article7. Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007. 25:123–129.8. Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. 2005. 568:653–663.
Article9. Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005. 568:291–303.
Article10. Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008. 29:268–275.
Article11. Manning SM, Talos DM, Zhou C, Selip DB, Park HK, Park CJ, Volpe JJ, Jensen FE. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 2008. 28:6670–6678.
Article12. Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005. 22:495–504.
Article13. Merzagora AC, Foffani G, Panyavin I, Mordillo-Mateos L, Aguilar J, Onaral B, Oliviero A. Prefrontal hemodynamic changes produced by anodal direct current stimulation. Neuroimage. 2010. 49:2304–2310.
Article14. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20:84–91.
Article15. Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995. 26:627–634.16. Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997. 28:2060–2065.
Article17. Kim MW, Bang MS, Han TR, Ko YJ, Yoon BW, Kim JH, Kang LM, Lee KM, Kim MH. Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain Res. 2005. 1052:16–21.
Article18. Ding Y, Li J, Lai Q, Rafols JA, Luan X, Clark J, Diaz FG. Motor balance and coordination training enhances functional outcome in rat with transient middle cerebral artery occlusion. Neuroscience. 2004. 123:667–674.
Article19. Schabitz WR, Li F, Fisher M. The N-methyl-D-aspartate antagonist CNS 1102 protects cerebral gray and white matter from ischemic injury following temporary focal ischemia in rats. Stroke. 2000. 31:1709–1714.20. Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, Loscher W, Paulus W, Tergau F. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006. 47:1216–1224.
Article21. Liebetanz D, Fregni F, Monte-Silva KK, Oliveira MB, Amancio-dos-Santos A, Nitsche MA, Guedes RC. After-effects of transcranial direct current stimulation (tDCS) on cortical spreading depression. Neurosci Lett. 2006. 398:85–90.
Article22. Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006. 117:1623–1629.
Article23. Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008. 115:289–296.
Article24. Kim DY, Park SH, Lee SU, Choi DH, Park HW, Paek SH, Shin HY, Kim EY, Park SP, Lim JH. Effect of human embryonic stem cell-derived neuronal precursor cell transplantation into the cerebral infarct model of rat with exercise. Neurosci Res. 2007. 58:164–175.
Article25. Wang RY, Yu SM, Yang YR. Treadmill training effects in different age groups following middle cerebral artery occlusion in rats. Gerontology. 2005. 51:161–165.
Article26. Church J, Zeman S. Ketamine promotes hippocampal CA1 pyramidal neuron loss after a short-duration ischemic insult in rats. Neurosci Lett. 1991. 123:65–68.
Article27. Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003. 553:293–301.
Article28. Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol. 2009. 120:1161–1167.
Article29. Ridenour TR, Warner DS, Todd MM, Baker MT. Effects of ketamine on outcome from temporary middle cerebral artery occlusion in the spontaneously hypertensive rat. Brain Res. 1991. 565:116–122.
Article30. Church J, Zeman S. Ketamine promotes hippocampal CA1 pyramidal neuron loss after a short-duration ischemic insult in rats. Neurosci Lett. 1991. 123:65–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Non-invasive Brain Stimulation on Dysphagia after Stroke
- Stroke Update 2011: Stroke Rehabilitation
- Non-Invasive Brain Stimulation for Treatment of Focal Hand Dystonia: Update and Future Direction
- Therapeutic Application of Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation in Depression
- Noninvasive brain stimulation: repetitive transcranial magnetic stimulation and transcranial direct current stimulation