J Korean Med Sci.
2006 Feb;21(1):25-29. 10.3346/jkms.2006.21.1.25.
Aprotinin Attenuates the Elevation of Pulmonary Vascular Resistance After Cardiopulmonary Bypass
- Affiliations
-
- 1Division of Pediatric Cardiac Surgery, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea. tjyun@amc.seoul.kr
- 2Department of Thoracic and Cardiovascular Surgery, Seoul National University, College of Medicine, Seoul, Korea.
- KMID: 2157777
- DOI: http://doi.org/10.3346/jkms.2006.21.1.25
Abstract
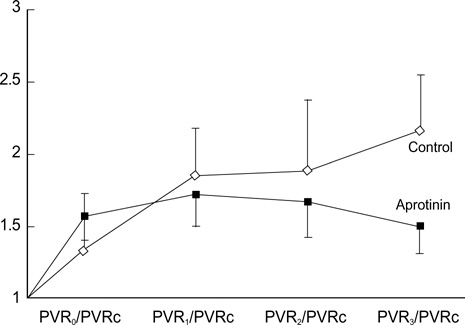
- Pulmonary vascular resistance (PVR) is generally believed to be elevated after cardiopulmonary bypass (CPB) due to whole body inflammation. Aprotinin has an antiinflammatory action, and it was hypothesized that aprotinin would attenuate the PVR increase induced by CPB. Ten mongrel dogs were placed under moderately hypothermic CPB for 2 hr. The experimental animals were divided into a control group (n=5, group I) and an aprotinin group (n=5, group II). In group II, aprotinin was administered during pre-bypass (50,000 KIU/kg) and post-bypass (10,000 KIU/kg) periods. Additional aprotinin (50,000 KIU/kg) was mixed in CPB priming solution. PVRs at pre-bypass and post-bypass 0, 1, 2, 3 hr were calculated, and lung tissue was obtained after the experiment. Post-bypass PVRs were significantly higher than prebypass levels in all animals (n=10, p<0.001). PVR elevation in group II was less than in group I at 3 hr post-bypass (p=0.0047). Water content of the lung was lower in group II (74+/-9.4%) compared to that of group I (83+/-9.5%), but the difference did not reach significance (p=0.076). Pathological examination showed a near normal lung structure in group II, whereas various inflammatory reactions were observed in group I. We concluded that aprotinin may attenuate CPB-induced PVR elevation through its anti-inflammatory effect.
MeSH Terms
Figure
Reference
-
1. Westaby S. Aprotinin in perspective. Ann Thorac Surg. 1993. 55:1033–1041.
Article2. Tweddell JS, Berger S, Frommelt PC, Pelech AN, Lewis DA, Fedderly RT, Frommelt MA, McManus TS, Mussatto KA, Kessel MW, Litwin SB. Aprotinin improves outcome of single-ventricle palliation. Ann Thorac Surg. 1996. 62:1329–1336.
Article3. Lu H, Soria C, Commin PL, Soria J, Piwnica A, Schumann F, Regnier O, Legrand Y, Caen JP. Hemostasis in patients undergoing extracorporeal circulation: the effect of aprotinin (Trasylol). Thromb Haemost. 1991. 66:633–637.
Article4. Huang H, Ding W, Su Z, Zhang W. Mechanism of the preserving effect of aprotinin on platelet function and its use in cardiac surgery. J Thorac Cardiovasc Surg. 1993. 106:11–18.
Article5. Komai H, Adatia IT, Elliott MJ, de Leval MR, Haworth SG. Increased plasma levels of endothelin-1 after cardiopulmonary bypass in patients with pulmonary hypertension and congenital heart disease. J Thorac Cardiovasc Surg. 1993. 106:473–478.
Article6. Rinder CS, Gaal D, Student LA, Smith BR. Platelet-leukocyte activation and modulation of adhesion receptors in pediatric patients with congenital heart disease undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1994. 107:280–288.
Article7. Bando K, Pillai R, Cameron DE, Brawn JD, Winkelsteins JA, Hutchin GM, Reitz BA, Baumgartner WA. Leukocyte depletion ameliorates free radical-mediated lung injury after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1990. 99:873–877.
Article8. Gillinov AM, Redmond JM, Zehr KJ, Wilson IC, Curtis WE, Bator JM, Burch RM, Reitz BA, Baumgartner WA, Herskowitz A. Inhibition of neutrophil adhesion during cardiopulmonary bypass. Ann Thorac Surg. 1994. 57:126–133.
Article9. Utley JR. Pathophysiology of cardiopulmonary bypass: Current Issues. J Card Surg. 1990. 5:177–189.
Article10. Cave AC, Marche A, Derias NW, Hearse DJ. Thromboxane A2 mediates pulmonary hypertension after cardiopulmonary bypass in the rabbit. J Thorac Cardiovasc Surg. 1993. 106:959–967.
Article11. Zehr KJ, Poston RS, Lee PC, Uthoff K, Kumar P, Cho PW, Gillinov AM, Redmond JM, Winkelstein JA, Herskowitz A. Platelet activating factor inhibition reduces lung injury after cardiopulmonary bypass. Ann Thorac Surg. 1995. 59:328–335.
Article12. Bourbon A, Vionnet M, Leprince P, Vaissier E, Copeland J, McDonagh P, Debre P, Gandjbakhch I. The effect of methylprednisolone treatment on the cardiopulmonary bypass-induced systemic inflammatory response. Eur J Cardiothorac Surg. 2004. 26:932–938.
Article13. Celik JB, Gormus N, Okesli S, Gormus ZI, Solak H. Methylprednisolone prevents inflammatory reaction occurring during cardiopulmonary bypass: effects on TNF-alpha, IL-6, IL-8, IL-10. Perfusion. 2004. 19:185–191.14. Chew MS, Brix-Christensen V, Ravn HB, Brandslund I, Ditlevsen E, Pedersen J, Hjortholm K, Hansen OK, Tonnesen E, Hjortdal VE. Effect of modified ultrafiltration on the inflammatory response in paediatric open-heart surgery: a prospective, randomized study. Perfusion. 2002. 17:327–333.15. Shimpo H, Shimamoto A, Sawamura Y, Fujinaga K, Kanemitsu S, Onoda K, Takao M, Mitani Y, Yada I. Ultrafiltration of the priming blood before cardiopulmonary bypass attenuates inflammatory response and improves postoperative clinical course in pediatric patients. Shock. 2001. 16:51–54.
Article16. Allison PM, Whitten CW. What is the mechanism of action of aprotinin? Anesthesiology. 1991. 75:377–379.
Article17. Boyle EM Jr, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovaslcuar surgery: the systemic inflammatory response. Ann Thorac Surg. 1997. 63:277–284.18. Royston D, Bidstrup BP, Sapsford RN, Taylor KM. Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet. 1987. 2:1289–1291.
Article19. Bidstrup BP, Royston D, Sapsford RN, Taylor KM. Reduction in blood loss and blood use after cardiopulmonary bypass with high dose aprotinin (Trasylol). J Thorac Cardiovasc Surg. 1989. 97:364–372.
Article20. Boldt J, Knothe C, Zickermann B, Wege N, Dapper F, Hempelmann G. Comparison of two aprotinin dosage regimens in pediatric patients having cardiac operations; Influence on platelet function and blood loss. J Thorac Cardiovasc Surg. 1993. 105:705–711.21. HerynKopf F, Lucchese F, Pereira E, Kalil R, Prates P, Nesralla IA. Aprotinin in children undergoing correction of congenital heart defects. A double-blind pilot study. J Thorac Cardiovasc Surg. 1994. 108:517–521.22. Penkoske PA, Entwistle LM, Marchak BE, Seal RF, Gibb W. Aprotinin in children undergoing repair of congenital heart defects. Ann Thorac Surg. 1995. 60:S529–S532.
Article23. Hill GE, Pohorecki R, Alonso A, Rennard SI, Robbins RA. Aprotinin reduces interleukin-8 production and lung neutrophil accumulation after cardiopulmonary bypass. Anesth Analg. 1996. 83:696–700.
Article24. Himmelfarb J, Holbrook D, McMonagle E. Effects of aprotinin on complement and granulocyte activation during ex vivo hemodialysis. Am J Kidney Dis. 1994. 24:901–906.
Article25. Wachtfogel YT, Kucich U, Hack CE, Gluszko P, Niewiarowski S, Colman RW, Edmunds LH Jr. Aprotinin inhibits the contact, neutrophil, and platelet activation systems during simulated extracoporeal perfusion. J Thorac Cardiovasc Surg. 1993. 106:1–10.26. Ali M, Becket J, Brannan J, Fleming J, Taylor KM. The effect of high dose aprotinin therapy on the systemic inflammatory response in a porcine model of cardiopulmonary bypass. Perfusion. 1996. 11:278–280.
Article27. Blauhut B, Gross C, Necek S, Doran JE, Spath P, Lundsgaard-Hansen P. Effects of high-dose aprotinin on blood loss, platelet function, fibrinolysis, complement, and renal function after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1991. 101:958–967.
Article28. Hill GE, Springall DR, Robbins RA. Aprotinin is associated with a decrease in nitric oxide production during cardiopulmonary bypass. Surgery. 1997. 121:449–455.
Article29. Boldt J, Osmer C, Linke LC, Dapper F, Hempelmann G. Circulating adhesion molecules in pediatric cardiac surgery. Anesth Analg. 1995. 81:1129–1135.
Article30. Aoki M, Jonas RA, Nomura F, Stromski ME, Tsuji MK, Hickey PR, Holtzman DH. Effects of aprotinin on acute recovery of cerebral metabolism in piglets after hypothermic circulatory arrest. Ann Thorac Surg. 1994. 58:146–153.
Article31. Lim C, Yun TJ, Kim YS, Kim SH, Lee JD, Rho JR, Song MG. Effect of aprotinin on changes in plasma thromboxane B2 and endothelin-1 concentration after extracorporeal circulation. Korean J Thorac Cardiovasc Surg. 2000. 33:221–230.32. Carrel TP, Schwanda M, Vogt PR, Turina MI. Aprotinin in pediatric cardiac operations: a benefit in complex malformations and with high-dose regimen only. Ann Thorac Surg. 1998. 66:153–158.
Article33. Dietrich W, Mossinger H, Spannagl M, Jochum M, Wendt P, Barankay A, Meisner H, Richter JA. Hemostatic activation during cardiopulmonary bypass with different aprotinin dosages in pediatric patients having cardiac operations. J Thorac Cardiovasc Surg. 1993. 105:712–720.
Article34. Kern FH, Morana NJ, Sears JJ, Hickey PR. Coagulation defects in neonate during cardiopulmonary bypass. Ann Thorac Surg. 1992. 54:541–546.35. Mcdonough J, Gruenwald C. The use of aprotinin in pediatric patients: a review. J Extra Corpor Technol. 2003. 35:346–349.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hemostatic Effects of High-Dose Aprotinin in Open Heart Operations
- Cardiac Pump Failure due to Inappropriate Heparinization: A case report
- Effect of Aprotinin on Changes in Plasma Thromboxane B2 and Endothelin-1 Concentratin after Extracorporeal Circulation
- Intraoperative Anaphylactoid Reaction Due to Aprotinin during Pediatric Open Heart Surgery
- Effects of Aprotinin and LPR in Priming Solution on the Inflammatory Reaction and Pulmonary Function after Cardiopulmonary Bypass