Ann Dermatol.
2008 Dec;20(4):179-183. 10.5021/ad.2008.20.4.179.
The Effect of 0.5% Sodium Tetradecyl Sulfate on a Venous Lake Lesion
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Hallym University, Anyang, Korea. 937121@hallym.or.kr
- KMID: 2156389
- DOI: http://doi.org/10.5021/ad.2008.20.4.179
Abstract
-
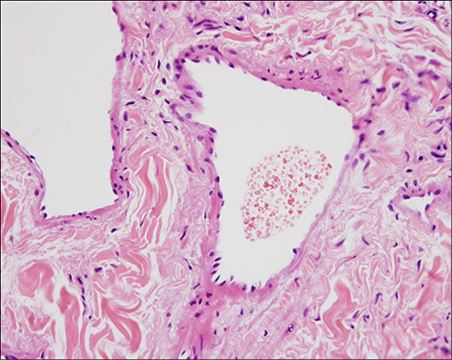
BACKGROUND: A venous lake lesion is a venous ectasia that occurs on the exposed skin of elderly people. Although a number of therapies such as surgical excision, laser therapy, infrared coagulation, cryotherapy and sclerotherapy have been used to treat venous lakes, there is no guideline for treating this lesion.
OBJECTIVE
The purpose of this study was to determine whether 0.5% sodium tetradecyl sulfate (STS) is effective for the treatment of venous lake lesions.
METHODS
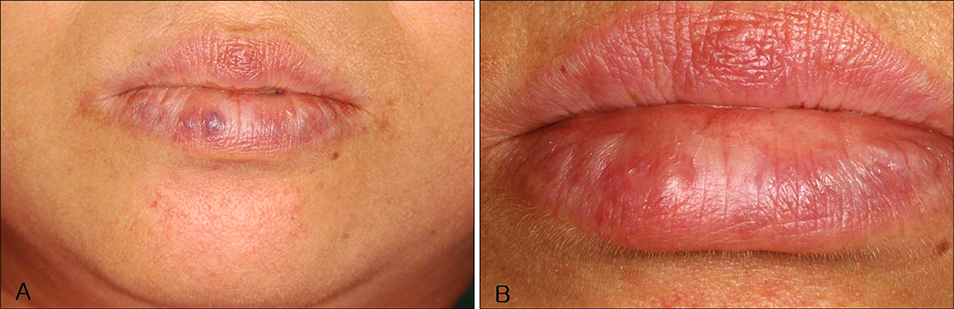
Twelve patients with venous lake lesions were enrolled In this study. After proper antiseptic preparation, 0.5% STS was slowly injected into each subject's lesion, and this was followed by immediate compression for 10 minutes.
RESULTS
After treatment, all of the patients' lesions cleared completely. The average number of treatments was 2.15+/-1.28. Two patients experienced mild side effects such as light pain and paresthesia, and these soon disappeared. There were no serious side effects reported during treatment. The mean follow up period was 29.58+/-13.48 months.
CONCLUSION
We have demonstrated that sclerotherapy with 0.5% STS was quite effective for treating venous lake lesions, and this treatment caused no serious adverse effects.
MeSH Terms
Figure
Reference
-
1. Landthaler M, Haina D, Waidelich W, Braun-Falco O. Laser therapy of venous lakes (Bean-Walsh) and telangiectasias. Plast Reconstr Surg. 1984; 73:78–83.
Article2. Bean WB, Walsh JR. Venous lakes. AMA Arch Derm. 1956; 74:459–463.
Article3. Alcalay J, Sandbank M. The ultrastructure of cutaneous venous lakes. Int J Dermatol. 1987; 26:645–646.
Article4. Bu J, Shi H, Hu M, Liu H. Oral venous lakes: a clinicopathologic analysis of 20 cases. Zhonghua Kou Qiang Yi Xue Za Zhi. 2002; 37:33–35.5. Gonzalez E, Gange RW, Momtaz KT. Treatment of telangiectases and other benign vascular lesions with the 577 nm pulsed dye laser. J Am Acad Dermatol. 1992; 27:220–226.
Article6. Neumann RA, Knobler RM. Venous lakes (Bean-Walsh) of the lips--treatment experience with the argon laser and 18 months follow-up. Clin Exp Dermatol. 1990; 15:115–118.
Article7. Colver GB, Hunter JA. Venous lakes: treatment by infrared coagulation. Br J Plast Surg. 1987; 40:451–453.
Article8. Suhonen R, Kuflik EG. Venous lakes treated by liquid nitrogen cryosurgery. Br J Dermatol. 1997; 137:1018–1019.
Article9. Kim WH, Kim SS, Kim CW, Kim KH, Kim KJ. Two cases of venous lake treated with a sclerosing agent. Korean J Dermatol. 2005; 43:71–73.10. Kuo HW, Yang CH. Venous lake of the lip treated with a sclerosing agent: report of two cases. Dermatol Surg. 2003; 29:425–428.
Article11. Winter H, Drager E, Sterry W. Sclerotherapy for treatment of hemangiomas. Dermatol Surg. 2000; 26:105–108.
Article12. de Lorimier AA. Sclerotherapy for venous malformations. J Pediatr Surg. 1995; 30:188–193.
Article13. Seccia A, Salgarello M. Treatment of angiomas with sclerosing injection of hydroxypolyethoxydodecan. Angiology. 1991; 42:23–29.
Article14. Goldman MP, Kaplan RP, Oki LN, Cavender PA, Strick RA, Bennett RG. Sclerosing agents in the treatment of telangiectasia. Comparison of the clinical and histologic effects of intravascular polidocanol, sodium tetradecyl sulfate, and hypertonic saline in the dorsal rabbit ear vein model. Arch Dermatol. 1987; 123:1196–1201.
Article15. Elder DE, Elenitsas R, Johnson BL Jr, Murphy GF. Lever's histopathology of the skin. 9th ed. Philadelphia: Lippincott Williams & Wilkins;2005. p. 1025.16. Goldman MP, Weiss RA, Brody HJ, Coleman WP 3rd, Fitzpatrick RE. Treatment of facial telangiectasia with sclerotherapy, laser surgery, and/or electrodesiccation: a review. J Dermatol Surg Oncol. 1993; 19:899–906.
Article17. Jackson IT, Carreno R, Potparic Z, Hussain K. Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg. 1993; 91:1216–1230.18. Goldman MP, Bennett RG. Treatment of telangiectasia: a review. J Am Acad Dermatol. 1987; 17:167–182.
Article19. Weiss RA, Goldman MP. Advances in sclerotherapy. Dermatol Clin. 1995; 13:431–445.
Article20. Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's dermatology in general medicine. 6th ed. New York: McGraw-Hill;2003. p. 2552–2553.21. Frullini A, Cavezzi A. Sclerosing foam in the treatment of varicose veins and telangiectases: history and analysis of safety and complications. Dermatol Surg. 2002; 28:11–15.
Article22. Bong HW, Hann SK, Kim DI, Im SB. Clicically improved venous malformation by sclerotherapy. Korean J Dermatol. 1993; 31:992–998.23. Goldman MP, Bergan JJ. Sclerotherapy: treatment of varicose and telangiectatic leg veins. 3rd ed. St. Louis: Mosby;2001. p. 151–240.24. del Pozo J, Pena C, Garcia Silva J, Goday JJ, Fonseca E. Venous lakes: a report of 32 cases treated by carbon dioxide laser vaporization. Dermatol Surg. 2003; 29:308–310.
Article25. Bekhor PS. Long-pulsed Nd:YAG laser treatment of venous lakes: report of a series of 34 cases. Dermatol Surg. 2006; 32:1151–1154.
Article26. Mun JH, Yi JH, Park SH, Lee JS. Two cases of venous lakes. Korean J Dermatol. 2005; 43:849–851.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Effects of Sclerotherapy of Venous Lake on the Lip
- Two Cases of Venous Lake Treated with a Sclerosing Agent
- A Case of Linear Digital Mucous Cyst Successfully Treated with 1% Sodium Tetradecyl Sulfate Intralesional Proximal End Injection
- Sclerotherapy of benign oral vascular lesion with sodium tetradecyl sulfate: cases report
- Therapeutic Effect of Sclerotherapy on Venous Malformations