J Vet Sci.
2014 Sep;15(3):369-379. 10.4142/jvs.2014.15.3.369.
Prevalence and characteristics of Shiga toxin-producing Escherichia coli (STEC) from cattle in Korea between 2010 and 2011
- Affiliations
-
- 1Department of Microbiology, College of Veterinary Medicine and BK21 Program for Veterinary Science, Seoul National University, Seoul 151-742, Korea. yhp2738@korea.kr
- 2Laboratory of Avian Diseases, College of Veterinary Medicine and BK21 Program for Veterinary Science, Seoul National University, Seoul 151-742, Korea.
- 3Animal and Plant Quarantine Agency, Anyang 430-757, Korea.
- KMID: 2155620
- DOI: http://doi.org/10.4142/jvs.2014.15.3.369
Abstract
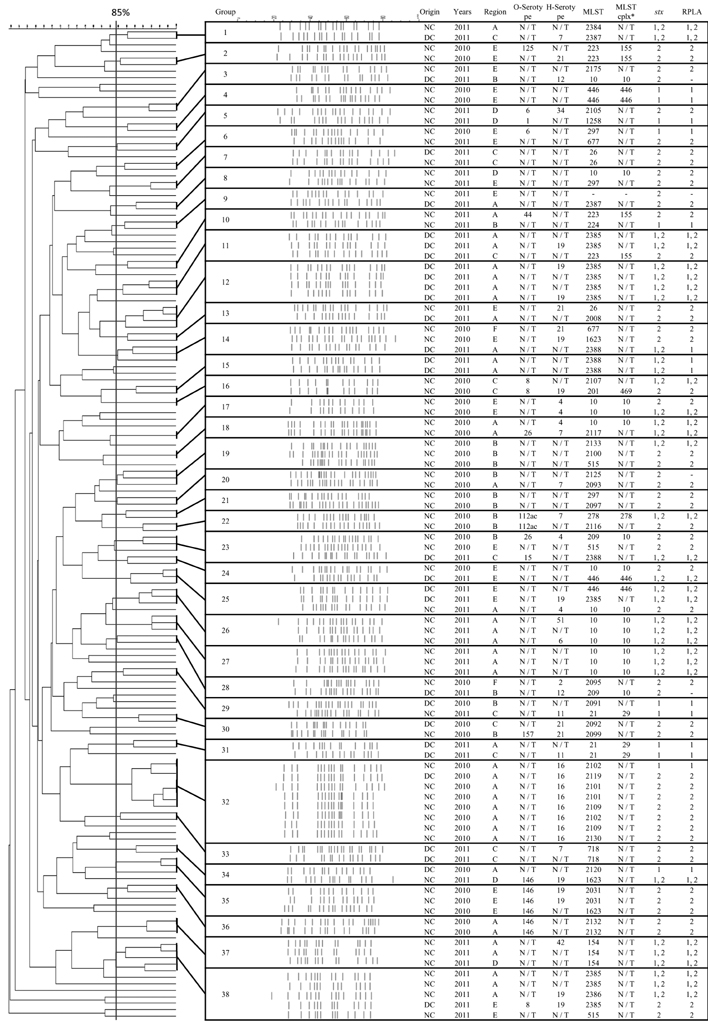
- A total of 156 Shiga-like toxin producing Escherichia coli (STEC) were isolated from fecal samples of Korean native (100/568, 18%) and Holstein dairy cattle (56/524, 11%) in Korea between September 2010 and July 2011. Fifty-two STEC isolates (33%) harbored both of shiga toxin1 (stx1) and shiga toxin2 (stx2) genes encoding enterohemolysin (EhxA) and autoagglutinating adhesion (Saa) were detected by PCR in 83 (53%) and 65 (42%) isolates, respectively. By serotyping, six STEC from native cattle and four STEC from dairy cattle were identified as O-serotypes (O26, O111, O104, and O157) that can cause human disease. Multilocus sequence typing and pulsed-field gel electrophoresis patterns highlighted the genetic diversity of the STEC strains and difference between strains collected during different years. Antimicrobial susceptibility tests showed that the multidrug resistance rate increased from 12% in 2010 to 42% in 2011. Differences between isolates collected in 2010 and 2011 may have resulted from seasonal variations or large-scale slaughtering in Korea performed to control a foot and mouth disease outbreak that occurred in early 2011. However, continuous epidemiologic studies will be needed to understand mechanisms. More public health efforts are required to minimize STEC infection transmitted via dairy products and the prevalence of these bacteria in dairy cattle.
Keyword
MeSH Terms
-
Animals
Anti-Bacterial Agents/pharmacology
Cattle/microbiology
Drug Resistance, Multiple, Bacterial
Electrophoresis, Gel, Pulsed-Field/veterinary
Escherichia coli Infections/epidemiology/microbiology/*veterinary
Female
Genes, Bacterial/genetics
Latex Fixation Tests/veterinary
Microbial Sensitivity Tests/veterinary
Multilocus Sequence Typing/veterinary
Prevalence
Republic of Korea/epidemiology
Shiga Toxin 1/genetics
Shiga Toxin 2/genetics
*Shiga-Toxigenic Escherichia coli/drug effects/genetics
Anti-Bacterial Agents
Shiga Toxin 1
Shiga Toxin 2
Figure
Reference
-
1. Barkocy-Gallagher GA, Arthur TM, Rivera-Betancourt M, Nou X, Shackelford SD, Wheeler TL, Koohmaraie M. Seasonal prevalence of Shiga toxin-producing Escherichia coli including O157:H7 and non-O157:H7 serotypes, and Salmonella in commercial beef processing plants. J Food Prot. 2003; 66:1978–1986.
Article2. Beutin L, Zimmermann S, Gleier K. Evaluation of the VTEC-Screen "Seiken" test for detection of different types of Shiga toxin (verotoxin)-producing Escherichia coli (STEC) in human stool samples. Diagn Microbiol Infect Dis. 2002; 42:1–8.
Article3. Blanco M, Blanco JE, Blanco J, Mora A, Prado C, Alonso MP, Mouriño M, Madrid C, Balsalobre C, Juárez A. Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet Microbiol. 1997; 54:309–319.
Article4. Chae HS, Kim NH, Han HJ, Son HR, Kim CK, Kim SH, Lee JH, Kim JT. Characterization and isolation of shiga toxin-producing Escherichia coli from bovine feces and carcass. Korean J Vet Serv. 2009; 32:241–249.5. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Informational Supplement M31-S1. Wayne: Clinical and Laboratory Standards Institute;2004.6. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. CLSI document M100-S17. Wayne: Clinical and Laboratory Standards Institute;2007.7. Cobbold R, Desmarchelier P. Horizontal transmission of Shiga toxin-producing Escherichia coli within groups of dairy calves. Appl Environ Microbiol. 2002; 68:4148–4152.
Article8. Cobbold RN, Rice DH, Szymanski M, Call DR, Hancock DD. Comparison of shiga-toxigenic Escherichia coli prevalences among dairy, feedlot, and cow-calf herds in Washington State. Appl Environ Microbiol. 2004; 70:4375–4378.
Article9. Donnenberg MS, Kaper JB, Finlay BB. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997; 5:109–114.
Article10. Foley SL, Simjee S, Meng J, White DG, McDermott PF, Zhao S. Evaluation of molecular typing methods for Escherichia coli O157:H7 isolates from cattle, food, and humans. J Food Prot. 2004; 67:651–657.
Article11. Fremaux B, Prigent-Combaret C, Vernozy-Rozand C. Long-term survival of Shiga toxin-producing Escherichia coli in cattle effluents and environment: an updated review. Vet Microbiol. 2008; 132:1–18.
Article12. Fukushima H, Seki R. High numbers of Shiga toxin-producing Escherichia coli found in bovine faeces collected at slaughter in Japan. FEMS Microbiol Lett. 2004; 238:189–197.
Article13. Gyles CL. Shiga toxin-producing Escherichia coli: an overview. J Anim Sci. 2007; 85:13 Suppl. E45–E62.14. Hong S, Oh KH, Cho SH, Kim JC, Park MS, Lim HS, Lee BK. Asymptomatic healthy slaughterhouse workers in South Korea carrying Shiga toxin-producing Escherichia coli. FEMS Immunol Med Microbiol. 2009; 56:41–47.
Article15. Hong S, Song SE, Oh KH, Kim SH, Yoo SJ, Lim HS, Park MS. Prevalence of farm and slaughterhouse vorkers carrying Shiga toxin-producing Escherichia coli in Korea. Osong Public Health Res Perspect. 2011; 2:198–201.
Article16. Hussein HS. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J Anim Sci. 2007; 85:13 Suppl. E63–E72.17. Kobayashi H, Shimada J, Nakazawa M, Morozumi T, Pohjanvirta T, Pelkonen S, Yamamoto K. Prevalence and characteristics of shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl Environ Microbiol. 2001; 67:484–489.
Article18. Koo HJ, Kwak HS, Yoon SH, Woo GJ. Phylogenetic group distribution and prevalence of virulence genes in Escherichia coli isolates from food samples in South Korea. World J Microbiol Biotechnol. 2012; 28:1813–1816.
Article19. Lim KG, Kang MI, Kim SK, Nam KW, Park HJ, Park JR, Cho KO, Lee BJ. Identification and characterization of Shiga toxin-producing Escherichia coli isolated from diarrhea in calves. Korean J Vet Res. 2006; 46:135–142.20. Montenegro MA, Bülte M, Trumpf T, Aleksić S, Reuter G, Bulling E, Helmuth R. Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J Clin Microbiol. 1990; 28:1417–1421.
Article21. Mora A, Blanco JE, Blanco M, Alonso MP, Dhabi G, Echeita A, González EA, Bernárdez MI, Blanco J. Antimicrobial resistance of Shiga toxin (verotoxin)-producing Escherichia coli O157:H7 and non-O157 strains isolated from humans, cattle, sheep and food in Spain. Res Microbiol. 2005; 156:793–806.
Article22. Noller AC, McEllistrem MC, Stine OC, Morris JG Jr, Boxrud DJ, Dixon B, Harrison LH. Multilocus sequence typing reveals a lack of diversity among Escherichia coli O157:H7 isolates that are distinct by pulsed-field gel electrophoresis. J Clin Microbiol. 2003; 41:675–679.
Article23. O'Brien AD, Holmes RK. Shiga and Shiga-like toxins. Microbiol Rev. 1987; 51:206–220.24. Paton AW, Paton JC. Direct detection and characterization of Shiga toxigenic Escherichia coli by multiplex PCR for stx1, stx2, eae, ehxA, and saa. J Clin Microbiol. 2002; 40:271–274.25. Paton AW, Srimanote P, Woodrow MC, Paton JC. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect Immun. 2001; 69:6999–7009.
Article26. Paton AW, Woodrow MC, Doyle RM, Lanser JA, Paton JC. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J Clin Microbiol. 1999; 37:3357–3361.
Article27. Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin microbiol rev. 1998; 11:450–479.
Article28. Pitondo-Silva A, Minarini LA, Camargo IL, Darini AL. Clonal relationships determined by multilocus sequence typing among enteropathogenic Escherichia coli isolated in Brazil. Can J Microbiol. 2009; 55:672–679.
Article29. Pradel N, Livrelli V, De Champs C, Palcoux JB, Reynaud A, Scheutz F, Sirot J, Joly B, Forestier C. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J Clin Microbiol. 2000; 38:1023–1031.
Article30. Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006; 3:59–67.
Article31. Sabat G, Rose P, Hickey WJ, Harkin JM. Selective and sensitive method for PCR amplification of Escherichia coli 16S rRNA genes in soil. Appl Environ Microbiol. 2000; 66:844–849.
Article32. Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995; 63:1055–1061.
Article33. Schroeder CM, Zhao C, DebRoy C, Torcolini J, Zhao S, White DG, Wagner DD, McDermott PF, Walker RD, Meng J. Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle, swine, and food. Appl Environ Microbiol. 2002; 68:576–581.
Article34. Shiomi M, Togawa M, Fujita K, Murata R. Effect of early oral fluoroquinolones in hemorrhagic colitis due to Escherichia coli O157:H7. Pediatr Int. 1999; 41:228–232.
Article35. Urwin R, Maiden MC. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 2003; 11:479–487.
Article36. Vali L, Wisely KA, Pearce MC, Turner EJ, Knight HI, Smith AW, Amyes SG. High-level genotypic variation and antibiotic sensitivity among Escherichia coli O157 strains isolated from two Scottish beef cattle farms. Appl Environ Microbiol. 2004; 70:5947–5954.
Article37. Vu-Khac H, Cornick NA. Prevalence and genetic profiles of Shiga toxin-producing Escherichia coli strains isolated from buffaloes, cattle, and goats in central Vietnam. Vet Microbiol. 2008; 126:356–363.
Article38. Wells JG, Shipman LD, Greene KD, Sowers EG, Green JH, Cameron DN, Downes FP, Martin ML, Griffin PM, Ostroff SM, Potter ME, Tauxe RV, Wachsmuth IK. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J Clin Microbiol. 1991; 29:985–989.
Article39. Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006; 60:1136–1151.
Article40. Yu J, Kaper JB. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992; 6:411–417.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of Salmonella enterica and Shiga toxin-producing Escherichia coli in zoo animals from Chile
- Epidemiological Analysis of Shiga Toxin-producing E. coli Isolated in Gwangju, Korea, by Pulse-field Gel Electrophoresis
- Development of a multiplex loop-mediated isothermal amplification assay to detect shiga toxin-producing Escherichia coli in cattle
- A Case of a Shiga Toxin Producing Escherichia Coli
- Identification of Shiga Toxin-producing E. coli Isolated from Diarrhea Patients and Cattle in Gwangju Area, Korea