Korean J Radiol.
2015 Aug;16(4):810-820. 10.3348/kjr.2015.16.4.810.
CT Perfusion Imaging Can Predict Patients' Survival and Early Response to Transarterial Chemo-Lipiodol Infusion for Liver Metastases from Colorectal Cancers
- Affiliations
-
- 1PET/CT Center, Qilu Hospital, First Affiliated Hospital of Shandong University, Jinan 250012, China. jiankuihan99@163.com
- 2Department of Radiology, Affiliated Anhui Provincial Hospital of Anhui Medical University, Hefei 230001, China.
- KMID: 2155554
- DOI: http://doi.org/10.3348/kjr.2015.16.4.810
Abstract
OBJECTIVE
To prospectively evaluate the performance of computed tomography perfusion imaging (CTPI) in predicting the early response to transarterial chemo-lipiodol infusion (TACLI) and survival of patients with colorectal cancer liver metastases (CRLM).
MATERIALS AND METHODS
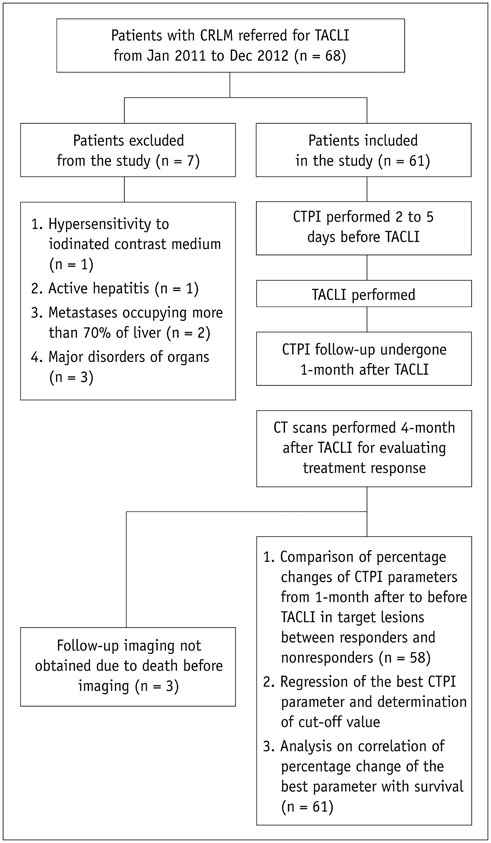
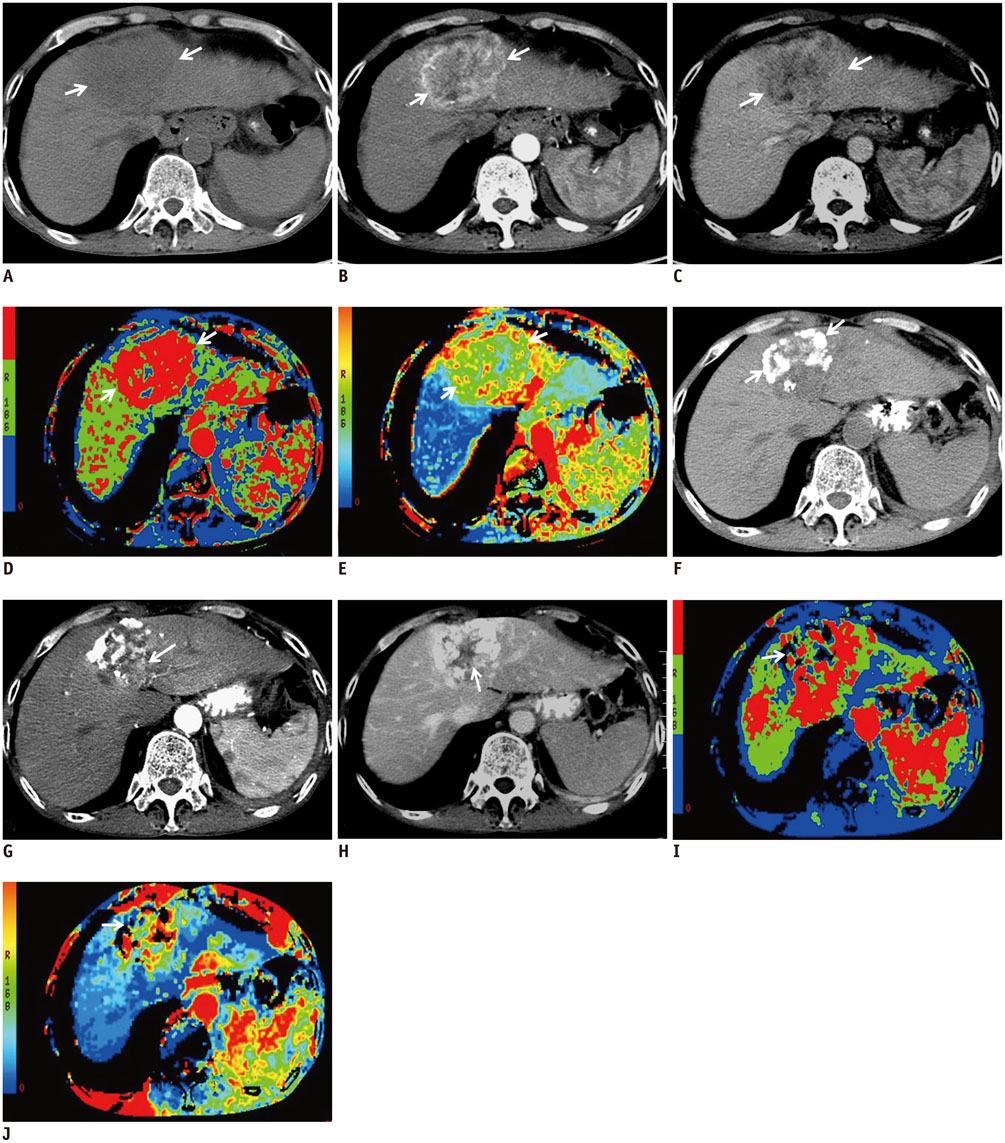
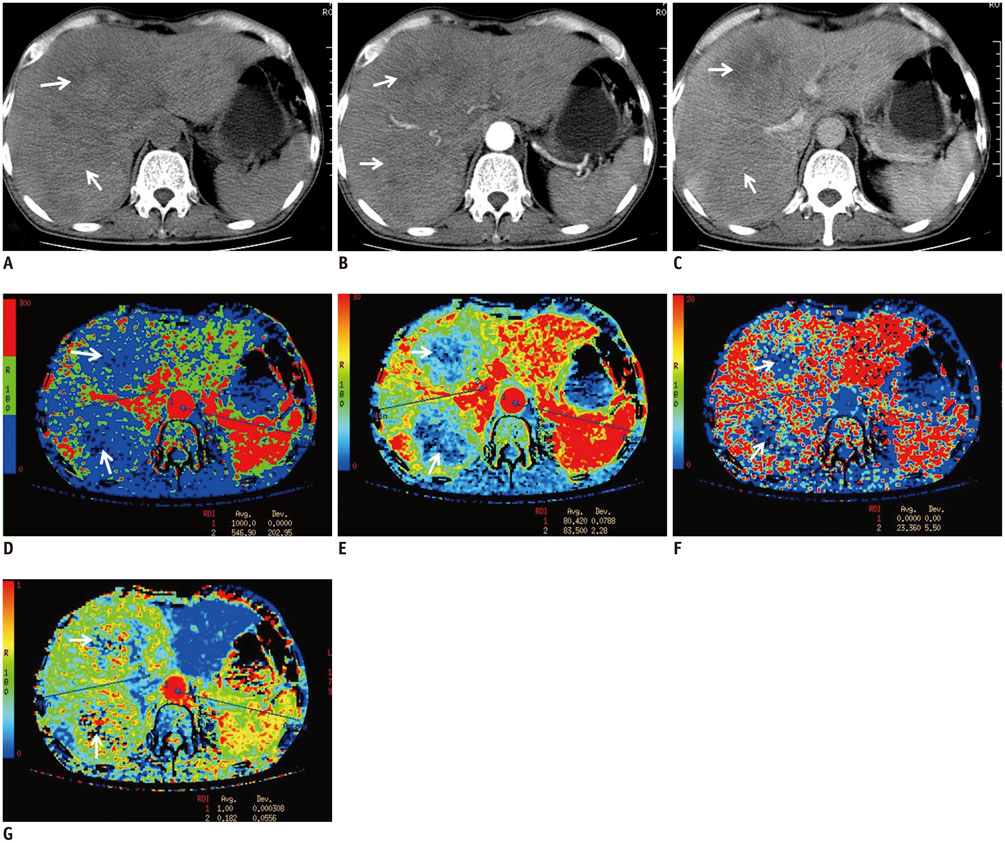
Computed tomography perfusion imaging was performed before and 1 month after TACLI in 61 consecutive patients. Therapeutic response was evaluated on CT scans 1 month and 4 months after TACLI; the patients were classified as responders and non-responders based on 4-month CT scans after TACLI. The percentage change of CTPI parameters of target lesions were compared between responders and non-responders at 1 month after TACLI. The optimal parameter and cutoff value were determined. The patients were divided into 2 subgroups according to the cutoff value. The log-rank test was used to compare the survival rates of the 2 subgroups.
RESULTS
Four-month images were obtained from 58 patients, of which 39.7% were responders and 60.3% were non-responders. The percentage change in hepatic arterial perfusion (HAP) 1 month after TACLI was the optimal predicting parameter (p = 0.003). The best cut-off value was -21.5% and patients who exhibited a > or = 21.5% decrease in HAP had a significantly higher overall survival rate than those who exhibited a < 21.5% decrease (p < 0.001).
CONCLUSION
Computed tomography perfusion imaging can predict the early response to TACLI and survival of patients with CRLM. The percentage change in HAP after TACLI with a cutoff value of -21.5% is the optimal predictor.
Keyword
MeSH Terms
-
Adult
Aged
Colorectal Neoplasms/mortality/*pathology
Contrast Media/administration & dosage
Ethiodized Oil/*administration & dosage
Female
Hepatic Artery/radiography
Humans
Liver Neoplasms/*drug therapy/mortality/*radiography/secondary
Male
Middle Aged
Perfusion Imaging/*methods
Prospective Studies
Survival Rate
Tomography, X-Ray Computed/methods
Contrast Media
Ethiodized Oil
Figure
Cited by 2 articles
-
Prediction of Treatment Outcome of Chemotherapy Using Perfusion Computed Tomography in Patients with Unresectable Advanced Gastric Cancer
Dong Ho Lee, Se Hyung Kim, Sang Min Lee, Joon Koo Han
Korean J Radiol. 2019;20(4):589-598. doi: 10.3348/kjr.2018.0306.Dynamic Contrast-Enhanced Ultrasound of Gastric Cancer: Correlation with Perfusion CT and Histopathology
Ijin Joo, Se Hyung Kim, Dong Ho Lee, Joon Koo Han
Korean J Radiol. 2019;20(5):781-790. doi: 10.3348/kjr.2018.0273.
Reference
-
1. Wang DS, Louie JD, Sze DY. Intra-arterial therapies for metastatic colorectal cancer. Semin Intervent Radiol. 2013; 30:12–20.2. Gruber-Rouh T, Naguib NN, Eichler K, Ackermann H, Zangos S, Trojan J, et al. Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer. 2014; 134:1225–1231.3. Leichman CG, Jacobson JR, Modiano M, Daniels JR, Zalupski MM, Doroshow JH, et al. Hepatic chemoembolization combined with systemic infusion of 5-fluorouracil and bolus leucovorin for patients with metastatic colorectal carcinoma: a Southwest Oncology Group pilot trial. Cancer. 1999; 86:775–781.4. Wilcox RA, Djulbegovic B, Moffitt HL, Guyatt GH, Montori VM. Randomized trials in oncology stopped early for benefit. J Clin Oncol. 2008; 26:18–19.5. Konopke R, Roth J, Volk A, Pistorius S, Folprecht G, Zöphel K, et al. Colorectal liver metastases: an update on palliative treatment options. J Gastrointestin Liver Dis. 2012; 21:83–91.6. Whisenant J, Venook A. Defining the role of hepatic arterial infusion chemotherapy in metastatic colorectal cancer. Oncology (Williston Park). 2004; 18:762–768. discussion 769-7737. Kennedy AS, Salem R. Radioembolization (yttrium-90 microspheres) for primary and metastatic hepatic malignancies. Cancer J. 2010; 16:163–175.8. Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009; 250:466–473.9. Li Z, Bonekamp S, Halappa VG, Corona-Villalobos CP, Pawlik T, Bhagat N, et al. Islet cell liver metastases: assessment of volumetric early response with functional MR imaging after transarterial chemoembolization. Radiology. 2012; 264:97–109.10. Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009; 115:616–623.11. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.12. Gowdra Halappa V, Corona-Villalobos CP, Bonekamp S, Li Z, Reyes D, Cosgrove D, et al. Neuroendocrine liver metastasis treated by using intraarterial therapy: volumetric functional imaging biomarkers of early tumor response and survival. Radiology. 2013; 266:502–513.13. Anzidei M, Napoli A, Zaccagna F, Cartocci G, Saba L, Menichini G, et al. Liver metastases from colorectal cancer treated with conventional and antiangiogenetic chemotherapy: evaluation with liver computed tomography perfusion and magnetic resonance diffusion-weighted imaging. J Comput Assist Tomogr. 2011; 35:690–696.14. Jiang T, Kambadakone A, Kulkarni NM, Zhu AX, Sahani DV. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST). Invest Radiol. 2012; 47:11–17.15. Yang L, Zhang XM, Zhou XP, Tang W, Guan YS, Zhai ZH, et al. Correlation between tumor perfusion and lipiodol deposition in hepatocellular carcinoma after transarterial chemoembolization. J Vasc Interv Radiol. 2010; 21:1841–1846.16. Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009; 250:281–289.17. Wang X, Xue HD, Jin ZY, Su BY, Li Z, Sun H, et al. Quantitative hepatic CT perfusion measurement: comparison of Couinaud's hepatic segments with dual-source 128-slice CT. Eur J Radiol. 2013; 82:220–226.18. Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010; 116:1305–1314.19. Islam R, Chyou PH, Burmester JK. Modeling efficacy of bevacizumab treatment for metastatic colon cancer. J Cancer. 2013; 4:330–335.20. Morsbach F, Sah BR, Spring L, Puippe G, Gordic S, Seifert B, et al. Perfusion CT best predicts outcome after radioembolization of liver metastases: a comparison of radionuclide and CT imaging techniques. Eur Radiol. 2014; 24:1455–1465.21. Reiner CS, Morsbach F, Sah BR, Puippe G, Schaefer N, Pfammatter T, et al. Early treatment response evaluation after yttrium-90 radioembolization of liver malignancy with CT perfusion. J Vasc Interv Radiol. 2014; 25:747–759.22. Morsbach F, Pfammatter T, Reiner CS, Fischer MA, Sah BR, Winklhofer S, et al. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial radioembolization of liver metastases. Invest Radiol. 2013; 48:787–794.23. Singh J, Sharma S, Aggarwal N, Sood RG, Sood S, Sidhu R. Role of perfusion CT differentiating hemangiomas from malignant hepatic lesions. J Clin Imaging Sci. 2014; 4:10.24. Kruskal JB, Kane RA. Pathophysiology of hepatic perfusion changes in livers containing small or occult metastases. Radiology. 1998; 209:291–292.25. Miles KA, Leggett DA, Kelley BB, Hayball MP, Sinnatamby R, Bunce I. In vivo assessment of neovascularization of liver metastases using perfusion CT. Br J Radiol. 1998; 71:276–281.26. Choi SH, Chung JW, Kim HC, Baek JH, Park CM, Jun S, et al. The role of perfusion CT as a follow-up modality after transcatheter arterial chemoembolization: an experimental study in a rabbit model. Invest Radiol. 2010; 45:427–436.27. Ng CS, Chandler AG, Wei W, Herron DH, Anderson EF, Kurzrock R, et al. Reproducibility of CT perfusion parameters in liver tumors and normal liver. Radiology. 2011; 260:762–770.28. Ippolito D, Sironi S, Pozzi M, Antolini L, Invernizzi F, Ratti L, et al. Perfusion CT in cirrhotic patients with early stage hepatocellular carcinoma: assessment of tumor-related vascularization. Eur J Radiol. 2010; 73:148–152.29. Ghanaati H, Mohammadzadeh V, Mohammadzadeh A, Firouznia K, Mohammadzadeh M, Motevali M, et al. Efficacy of transarterial chemoembolization on lesion reduction in colorectal liver metastases. Acta Med Iran. 2012; 50:535–540.30. Thng CH, Koh TS, Collins DJ, Koh DM. Perfusion magnetic resonance imaging of the liver. World J Gastroenterol. 2010; 16:1598–1609.31. Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007; 243:736–743.32. Jiang ZX, Peng WJ, Li WT, Tang F, Liu SY, Qu XD, et al. Effect of b value on monitoring therapeutic response by diffusion-weighted imaging. World J Gastroenterol. 2008; 14:5893–5899.33. Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011; 55:1309–1316.34. Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012; 262:708–771.35. Yeo DM, Choi JI, Lee YJ, Park MY, Chun HJ, Lee HG. Comparison of RECIST, mRECIST, and choi criteria for early response evaluation of hepatocellular carcinoma after transarterial chemoembolization using drug-eluting beads. J Comput Assist Tomogr. 2014; 38:391–397.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An A to Z of Lipiodol Beyond the Clinical Practice in the Management of Hepatocellular Carcinoma
- Comparison of the Response Evaluation Criteria in Solid Tumors with Volumetric Measurement for Evaluation of Response and Overall Survival with Liver Metastases from Colorectal Cancer
- Liver Metastases in Colorectal Cancer
- Radiomics and machine learning analysis of liver magnetic resonance imaging for prediction and early detection of tumor response in colorectal liver metastases
- Analysis of Survival Rates by Surgical Resection or Transarterial Chemo-embolization as the Initial Treatment for the Patients with Hepatocellular Carcinoma (UICC T stage 1, 2, 3) and Child-Pugh Class A Liver Function