Cancer Res Treat.
2016 Jan;48(1):312-321. 10.4143/crt.2014.266.
Cross-sectional Study of Patients with Diffuse Large B-Cell Lymphoma: Assessing the Effect of Host Status, Tumor Burden, and Inflammatory Activity on Venous Thromboembolism
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kstwoh@skku.edu
- 2Biostatistics Team, Samsung Biomedical Research Institute, Seoul, Korea.
- 3Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2152290
- DOI: http://doi.org/10.4143/crt.2014.266
Abstract
- PURPOSE
The risk factors for venous thromboembolism (VTE) in diffuse large B-cell lymphoma (DLBCL) are not clear although thrombosis can be associated with host status, tumor burden, and inflammatory activity. We assessed the effect of those factors on VTE in a cross-sectional study of patients enrolled in a prospective cohort study.
MATERIALS AND METHODS
We analyzed the occurrence of VTE in 322 patients with newly diagnosed DLBCL who received rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) between 2008 and 2011. Serum levels of inflammatory cytokines were measured from serum samples archived at diagnosis.
RESULTS
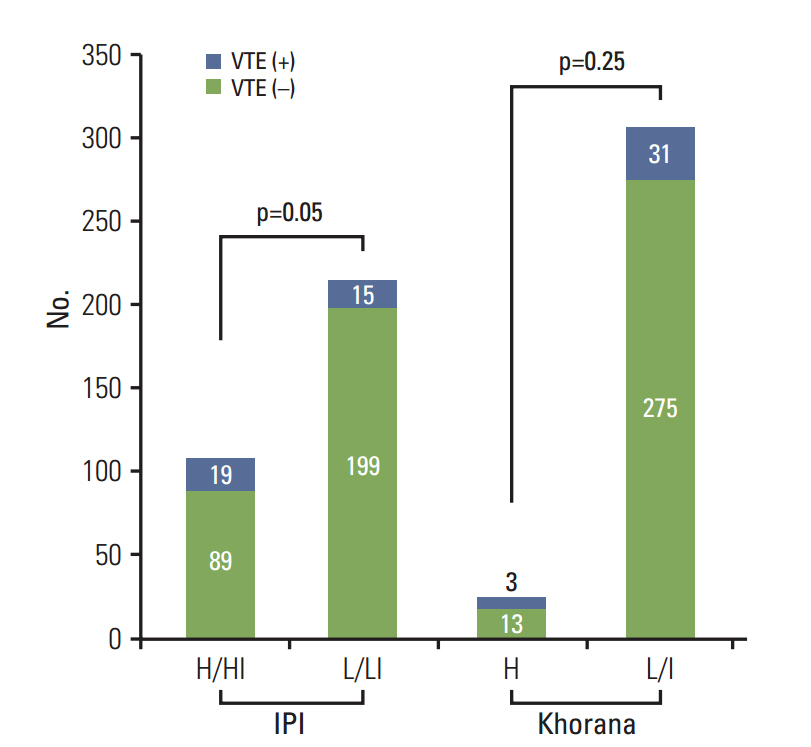
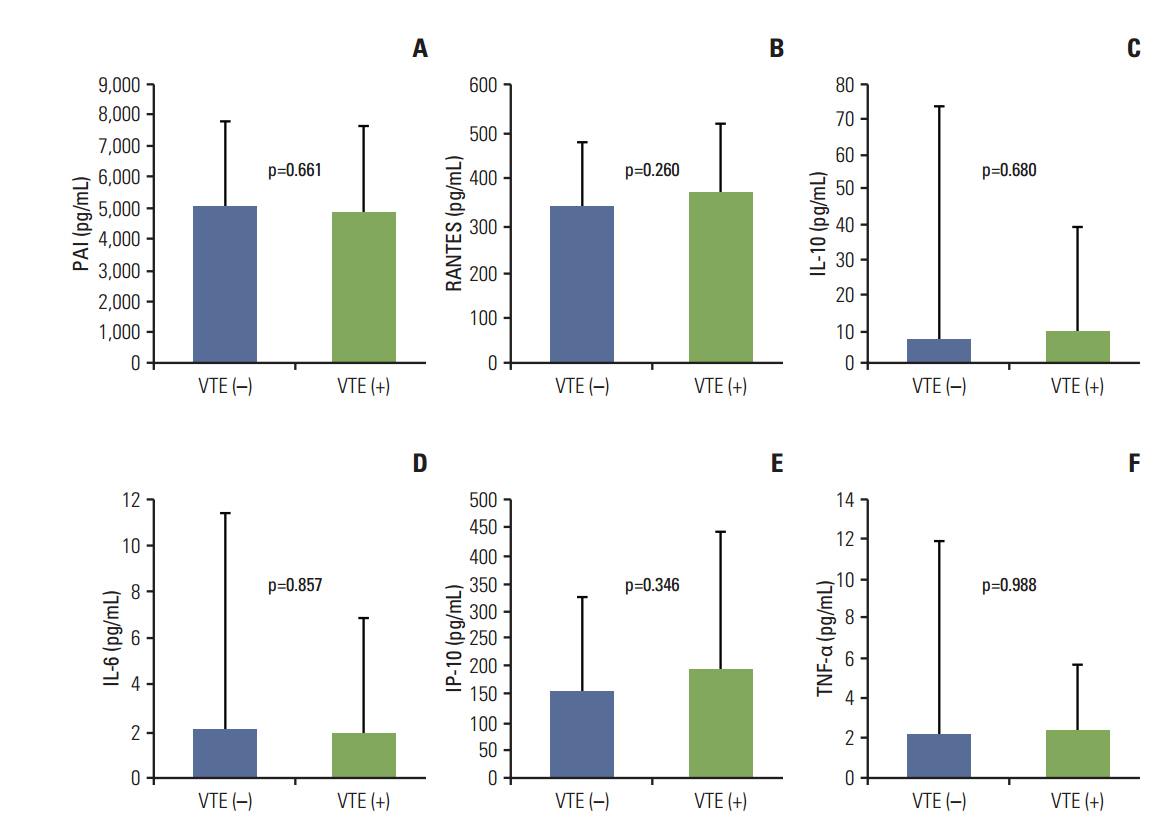
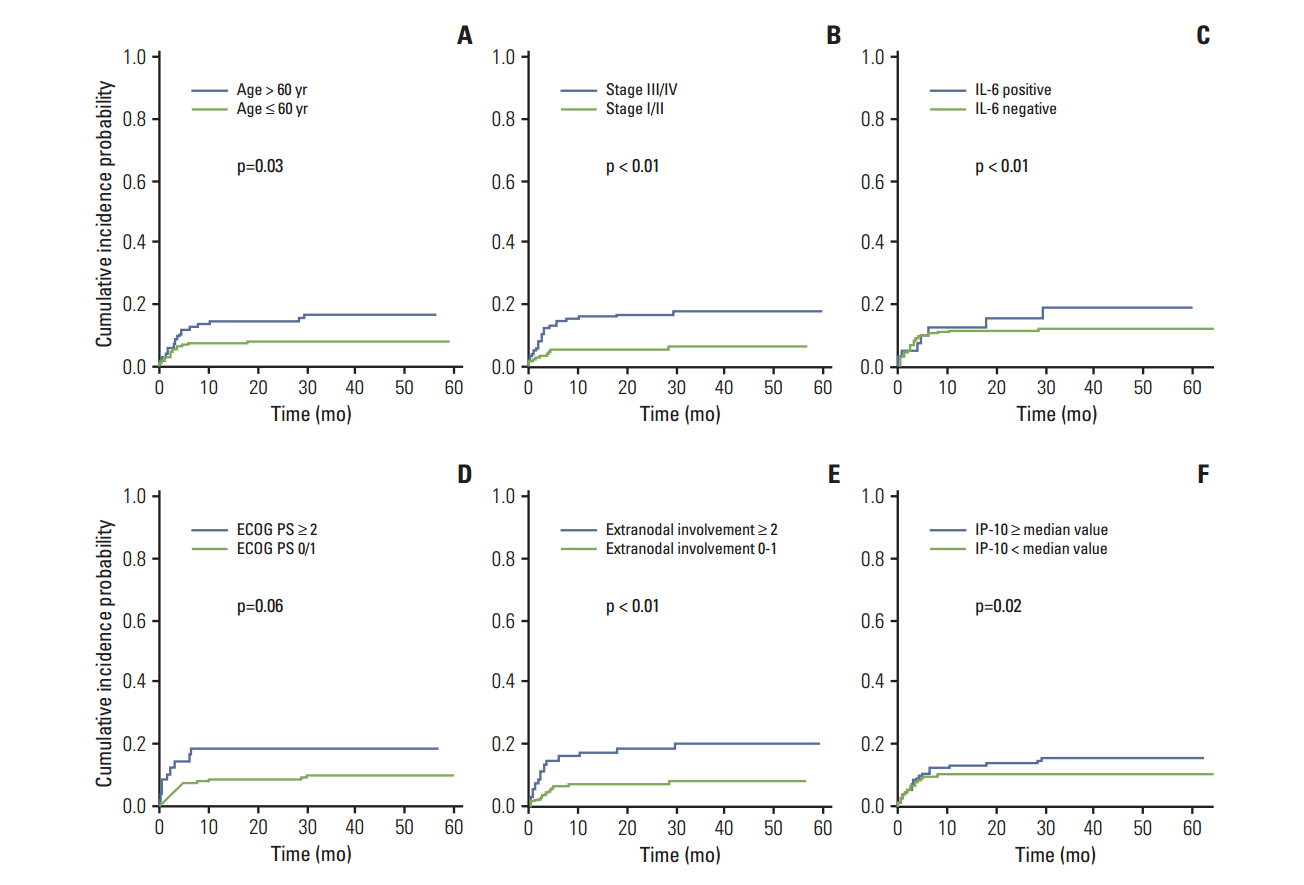
With a median follow-up duration of 41.9 months, VTE was documented in 34 patients (10.6%). A comparison of baseline characteristics indicated the group with VTE had higher percentage of old age, stage III/IV and extranodal involvements than the group without VTE (p < 0.05). Thus, the International Prognostic Index was significantly associated with VTE, but the Khorana score was not. A univariate competing risk factor analysis for VTE revealed that increased levels of inflammatory cytokines such as interleukin (IL)-6 and IL-10 were also associated with VTE (p < 0.05) in addition to host and tumor burden. However, a multivariate analysis showed that two host factors including age (> or = 60 years) and poor performance were independent risk factors for VTE.
CONCLUSION
Among potential risk factors for VTE including tumor burden and inflammatory activity, age and performance status had a strong impact on the occurrence of VTE in patients with DLBCL who received R-CHOP.
MeSH Terms
-
B-Lymphocytes*
Cohort Studies
Cross-Sectional Studies*
Cyclophosphamide
Cytokines
Diagnosis
Doxorubicin
Drug Therapy
Follow-Up Studies
Humans
Interleukin-10
Interleukins
Lymphoma, B-Cell*
Multivariate Analysis
Prednisone
Prospective Studies
Risk Factors
Thrombosis
Tumor Burden*
Venous Thromboembolism*
Vincristine
Cyclophosphamide
Cytokines
Doxorubicin
Interleukin-10
Interleukins
Prednisone
Vincristine
Figure
Reference
-
References
1. Lekovic D, Miljic P, Mihaljevic B. Increased risk of venous thromboembolism in patients with primary mediastinal large B-cell lymphoma. Thromb Res. 2010; 126:477–80.
Article2. Caruso V, Di Castelnuovo A, Meschengieser S, Lazzari MA, de Gaetano G, Storti S, et al. Thrombotic complications in adult patients with lymphoma: a meta-analysis of 29 independent cohorts including 18 018 patients and 1149 events. Blood. 2010; 115:5322–8.
Article3. Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012; 47:92–104.
Article4. Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005; 23:6387–93.
Article5. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–42.
Article6. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010; 116:2040–5.
Article7. Yokoyama K, Murata M, Ikeda Y, Okamoto S. Incidence and risk factors for developing venous thromboembolism in Japanese with diffuse large b-cell lymphoma. Thromb Res. 2012; 130:7–11.
Article8. Park LC, Woo SY, Kim S, Jeon H, Ko YH, Kim SJ, et al. Incidence, risk factors and clinical features of venous thromboembolism in newly diagnosed lymphoma patients: results from a prospective cohort study with Asian population. Thromb Res. 2012; 130:e6.
Article9. Ferroni P, Riondino S, Formica V, Cereda V, Tosetto L, La Farina F, et al. Venous thromboembolism risk prediction in ambulatory cancer patients: clinical significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio. Int J Cancer. 2015; 136:1234–40.
Article10. Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005; 6:401–10.
Article11. Reitsma PH, Rosendaal FR. Activation of innate immunity in patients with venous thrombosis: the Leiden Thrombophilia Study. J Thromb Haemost. 2004; 2:619–22.
Article12. Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005; 25:1512–8.13. Lv W, Duan Q, Wang L, Gong Z, Yang F, Song Y. Gene expression levels of cytokines in peripheral blood mononuclear cells from patients with pulmonary embolism. Mol Med Rep. 2013; 7:1245–50.
Article14. Komrokji RS, Uppal NP, Khorana AA, Lyman GH, Kaplan KL, Fisher RI, et al. Venous thromboembolism in patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2006; 47:1029–33.
Article15. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007; 110:2339–46.
Article16. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008; 111:4902–7.
Article17. Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005; 104:2822–9.
Article18. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996; 17:343–6.
Article19. Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010; 116:5377–82.
Article20. Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis: a systematic review of clinical studies. Thromb Haemost. 2005; 94:362–5.
Article21. van Aken BE, den Heijer M, Bos GM, van Deventer SJ, Reitsma PH. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost. 2000; 83:536–9.
Article22. Poredos P, Jezovnik MK. In patients with idiopathic venous thrombosis, interleukin-10 is decreased and related to endothelial dysfunction. Heart Vessels. 2011; 26:596–602.
Article23. Christiansen SC, Naess IA, Cannegieter SC, Hammerstrom J, Rosendaal FR, Reitsma PH. Inflammatory cytokines as risk factors for a first venous thrombosis: a prospective population-based study. PLoS Med. 2006; 3:e334.
Article24. Incalcaterra E, Meli F, Muratori I, Corrado E, Amato C, Canino B, et al. Residual vein thrombosis and onset of post-thrombotic syndrome: influence of the 4G/5G polymorphism of plasminogen activator inhibitor-1 gene. Thromb Res. 2014; 133:371–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relapse of Ocular Lymphoma following Primary Testicular Diffuse Large B-cell Lymphoma
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- The Expression of p16 in Diffuse Large B-cell Lymphoma and Its Prognostic Implications
- Inverse Psoriasis Developed in a Patient with Diffuse Large B Cell Lymphoma
- Relationships among Hepatitis C Virus, Hepatocellular Carcinoma, and Diffuse Large B Cell Lymphoma: A Case Report