Cancer Res Treat.
2016 Jan;48(1):162-170. 10.4143/crt.2015.017.
Severe Imatinib-Associated Skin Rash in Gastrointestinal Stromal Tumor Patients: Management and Clinical Implications
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ykkang@amc.seoul.kr
- 2Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2152272
- DOI: http://doi.org/10.4143/crt.2015.017
Abstract
- PURPOSE
This study evaluated the incidence of imatinib-associated skin rash, the interventional outcomes of severe rash, and impact of severe rash on the outcomes of imatinib treatment in gastrointestinal stromal tumor (GIST) patients.
MATERIALS AND METHODS
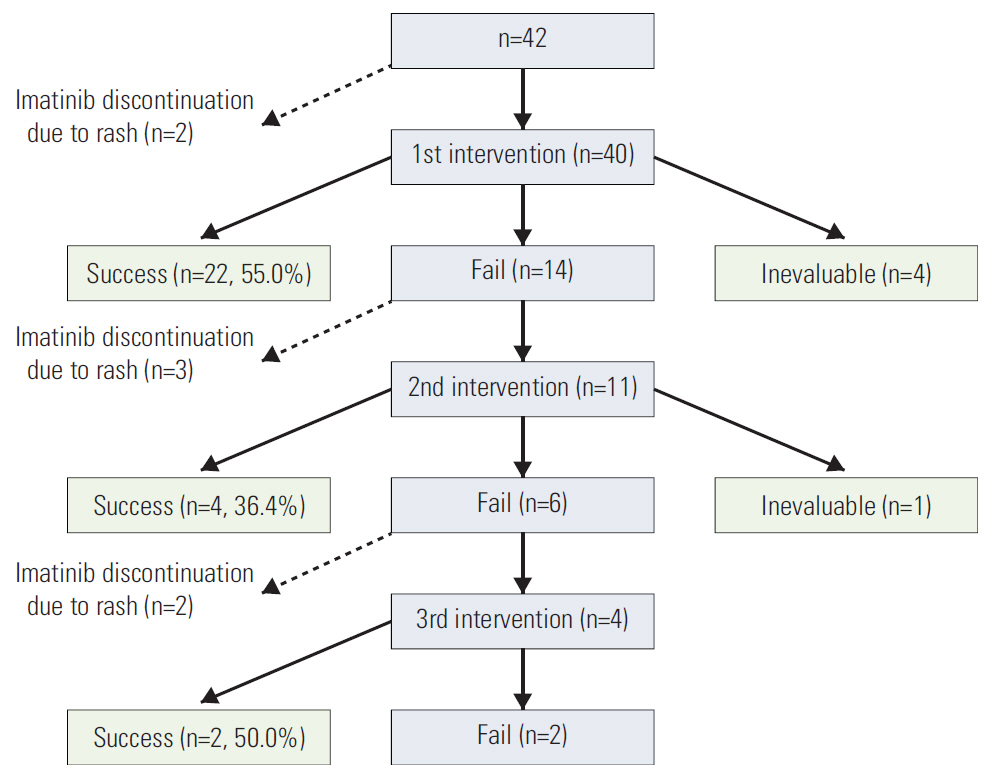
A total of 620 patients were administered adjuvant or palliative imatinib for GIST at Asan Medical Center between January 2000 and July 2012. This analysis focused on a group of 42 patients who developed a severe rash requiring major interventions, defined as dose interruption or reduction of imatinib or systemic steroid use.
RESULTS
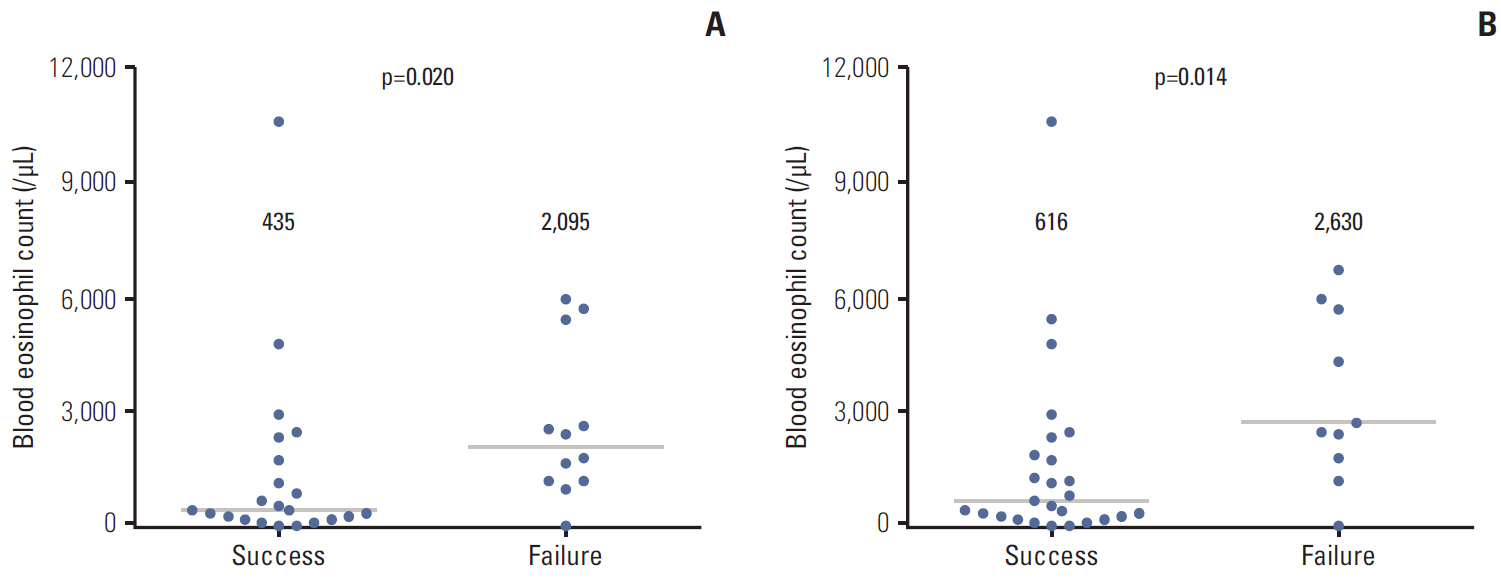
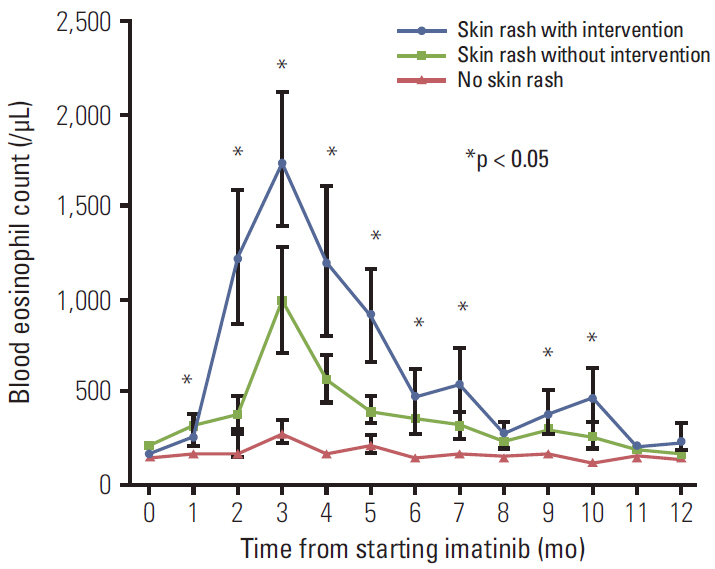
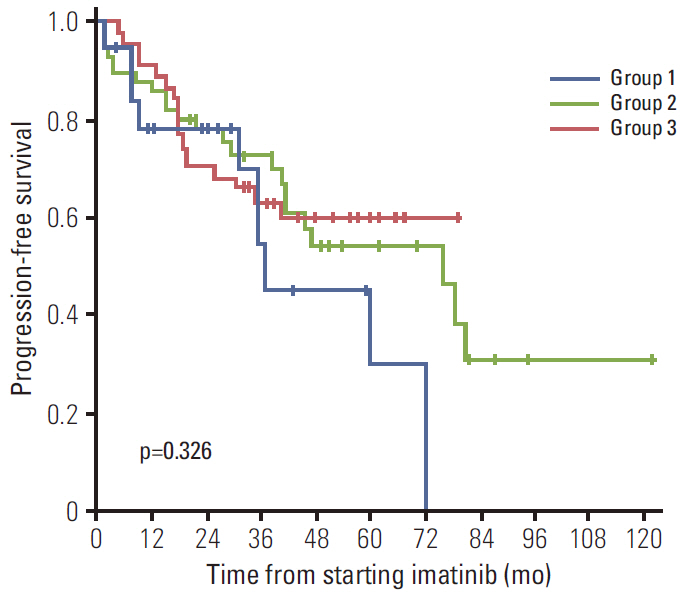
Of the 620 patients treated with imatinib, 148 patients (23.9%) developed an imatinib-associated skin rash; 42 patients (6.8%) developed a severe rash requiring major intervention. Of these, 28 patients (66.8%) successfully continued imatinib with interventions. Serial blood eosinophil levels during imatinib treatment were associated with skin rash and severity. A significant association was observed between successful intervention and blood eosinophil level at the time of intervention initiation. In metastatic settings, patients with severe rash requiring major interventions tended to show poorer progression-free survival than patients who did not require major intervention and patients with no rash, although this finding was not statistically significant (p=0.326).
CONCLUSION
By aggressive treatment of severe rash through modification of imatinib dose or use of systemic steroid, the majority of patients can continue on imatinib. In particular, imatinib dose intensity can be maintained with use of systemic steroid. Measuring the blood eosinophil levels may be helpful in guiding the management plan for skin rash regarding the intensity and duration of interventions.
MeSH Terms
Figure
Reference
-
References
1. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–80.
Article2. Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004; 364:1127–34.
Article3. Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008; 26:620–5.4. Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009; 373:1097–104.
Article5. Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schutte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012; 307:1265–72.6. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006; 368:1329–38.
Article7. Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381:295–302.
Article8. Kang YK, Ryu MH, Yoo C, Ryoo BY, Kim HJ, Lee JJ, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013; 14:1175–82.
Article9. ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23 Suppl 7:vii49–55.10. Kang YK, Kang HJ, Kim KM, Sohn T, Choi D, Ryu MH, et al. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat. 2012; 44:85–96.
Article11. Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010; 11:942–9.
Article12. Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009; 27:3141–7.
Article13. Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006; 24:4764–74.
Article14. Longley BJ, Carter EL. SCF-KIT pathway in human epidermal melanocyte homeostasis. J Invest Dermatol. 1999; 113:139–40.
Article15. Park HJ, Kim HS, Kim HJ, Lee JY, Cho BK, Lee AW, et al. Immunohistochemical characterization of cutaneous drug eruptions by STI571. J Dermatol Sci. 2005; 38:9–15.
Article16. Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013; 12:117–29.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Generalized Keratosis Pilaris Induced by Imatinib Mesylate
- Imatinib-induced DRESS Syndrome in Gastrointestinal Stromal Tumor
- Metastatic Cutaneous Duodenal Gastrointestinal Stromal Tumor: A Possible Clue to Multiple Metastases
- A Case of Disseminated Intra-abdominal Gastrointestinal Stromal Tumor Managed with Low Dose Imatinib
- Diagnosis and Treatment of Imatinib-induced Pneumonitis in a Patient with High-risk Rectal Gastrointestinal Stromal Tumor