Investig Magn Reson Imaging.
2015 Dec;19(4):218-223. 10.13104/imri.2015.19.4.218.
Application of Volumetric Analysis to Glioblastomas: a Correlation Study on the Status of the Isocitrate Dehydrogenase Mutation
- Affiliations
-
- 1Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Cancer Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- 5Department of Radiation Oncology, Cancer Research Institute, Seoul National University Hospital, Seoul, Korea.
- 6Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea. verocay@snuh.org
- 7Department of Radiology, Seoul National University Hospital, Seoul, Korea.
- 8Center for Nanoparticle Research, Institute for Basic Science (IBS), Seoul, Korea.
- 9School of Chemical and Biological Engineering, Seoul National University, Seoul, Korea.
- KMID: 2151768
- DOI: http://doi.org/10.13104/imri.2015.19.4.218
Abstract
- PURPOSE
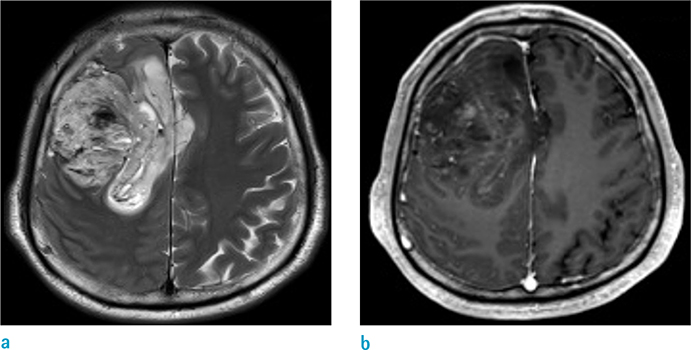
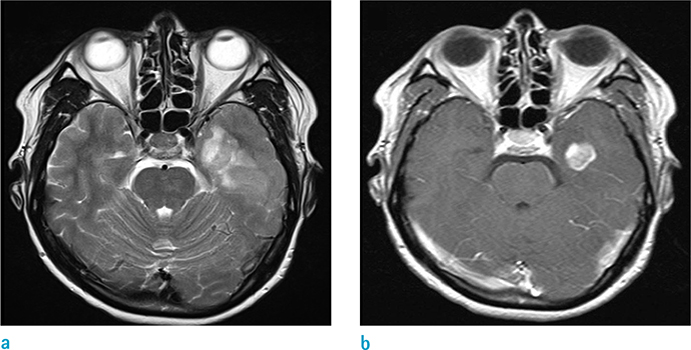
To investigate whether volumetric analysis based on T2WI and contrast-enhanced (CE) T1WI can distinguish between isocitrate dehydrogenase-1 mutation-positive (IDH1(P)) and -negative (IDH1(N)) glioblastomas (GBMs).
MATERIALS AND METHODS
We retrospectively enrolled 109 patients with histopathologically proven GBMs after surgery or stereotactic biopsy and preoperative MR imaging. We measured the whole-tumor volume in each patient using a semiautomatic segmentation method based on both T2WI and CE T1WI. We compared the tumor volumes between IDH1(P) (n = 12) and IDH1(N) (n = 97) GBMs using an unpaired t-test. In addition, we performed receiver operating characteristic (ROC) analysis for the differentiation of IDH1(P) and IDH1(N) GBMs using the tumor volumes based on T2WI and CE T1WI.
RESULTS
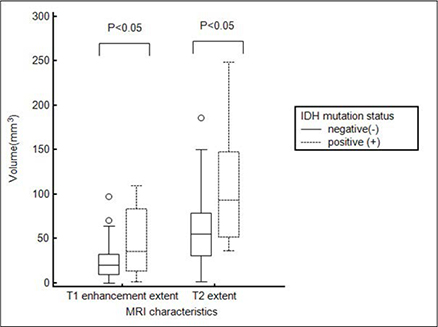
The mean tumor volume based on T2WI was larger for IDH1(P) GBMs than IDH1(N) GBMs (108.8 +/- 68.1 and 59.3 +/- 37.3 mm3, respectively, P = 0.0002). In addition, IDH1(P) GBMs had a larger tumor volume on CE T1WI than did IDH1(N) tumors (49.00 +/- 40.14 and 22.53 +/- 17.51 mm3, respectively, P < 0.0001). ROC analysis revealed that the tumor volume based on T2WI could distinguish IDH1(P) from IDH1(N) with a cutoff value of 90.25 (P < 0.05): 7 of 12 IDH1(P) (58.3%) and 79 of 97 IDH1(N) (81.4%).
CONCLUSION
Volumetric analysis of T2WI and CE T1WI could enable IDH1(P) GBMs to be distinguished from IDH1(N) GBMs. We assumed that secondary GBMs with IDH1(P) underwent stepwise progression and were more infiltrative than those with IDH1(N), which might have resulted in the differences in tumor volume.
MeSH Terms
Figure
Reference
-
1. Daumas-Duport C, Scheithauer B, O'Fallon J, Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer. 1988; 62:2152–2165.2. Pedersen CL, Romner B. Current treatment of low grade astrocytoma: a review. Clin Neurol Neurosurg. 2013; 115:1–8.3. Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008; 116:597–602.4. Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009; 15:6002–6007.5. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360:765–773.6. Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010; 120:707–718.7. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008; 321:1807–1812.8. Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009; 27:4150–4154.9. Levner I, Drabycz S, Roldan G, De Robles P, Cairncross JG, Mitchell R. Predicting MGMT methylation status of glioblastomas from MRI texture. Med Image Comput Comput Assist Interv. 2009; 12:522–530.10. Jenkinson MD, du Plessis DG, Smith TS, Joyce KA, Warnke PC, Walker C. Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain. 2006; 129:1884–1891.11. Aghi M, Gaviani P, Henson JW, Batchelor TT, Louis DN, Barker FG 2nd. Magnetic resonance imaging characteristics predict epidermal growth factor receptor amplification status in glioblastoma. Clin Cancer Res. 2005; 11:8600–8605.12. Mut M, Turba UC, Botella AC, Baskurt E, Lopes MB, Shaffrey ME. Neuroimaging characteristics in subgroup of GBMs with p53 overexpression. J Neuroimaging. 2007; 17:168–174.13. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845.14. Lee S, Choi SH, Ryoo I, et al. Evaluation of the micro-environmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J Neurooncol. 2015; 121:141–150.15. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013; 19:764–772.16. Paganetti PA, Caroni P, Schwab ME. Glioblastoma infiltration into central nervous system tissue in vitro: involvement of a metalloprotease. J Cell Biol. 1988; 107:2281–2291.17. Andronesi OC, Rapalino O, Gerstner E, et al. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest. 2013; 123:3659–3663.18. Pope WB, Prins RM, Albert Thomas M, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012; 107:197–205.19. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012; 18:624–629.20. SongTao Q, Lei Y, Si G, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012; 103:269–273.21. Tran AN, Lai A, Li S, et al. Increased sensitivity to radiochemotherapy in IDH1 mutant glioblastoma as demonstrated by serial quantitative MR volumetry. Neuro Oncol. 2014; 16:414–420.22. Carrillo JA, Lai A, Nghiemphu PL, et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol. 2012; 33:1349–1355.23. Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012; 3:709–722.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determination of serum isocitrate dehydrogenase activities in thedifferentation of contrilobular from periportal hepatic necrosis in rats
- Immunohistochemical Classification of Primary and Secondary Glioblastomas
- In-Vivo Proton Magnetic Resonance Spectroscopy of 2-Hydroxyglutarate in Isocitrate Dehydrogenase-Mutated Gliomas: A Technical Review for Neuroradiologists
- IDH Mutation Analysis in Ewing Sarcoma Family Tumors
- Identification of a New Selective Chemical Inhibitor of Mutant Isocitrate Dehydrogenase-1