J Pathol Transl Med.
2015 Jul;49(4):279-287. 10.4132/jptm.2015.06.11.
A Review of Inflammatory Processes of the Breast with a Focus on Diagnosis in Core Biopsy Samples
- Affiliations
-
- 1Department of Pathology and Laboratory Medicine, Weill Cornell Medical College, New York, NY, USA. tid9007@med.cornell.edu
- KMID: 2151137
- DOI: http://doi.org/10.4132/jptm.2015.06.11
Abstract
- Inflammatory and reactive lesions of the breast are relatively uncommon among benign breast lesions and can be the source of an abnormality on imaging. Such lesions can simulate a malignant process, based on both clinical and radiographic findings, and core biopsy is often performed to rule out malignancy. Furthermore, some inflammatory processes can mimic carcinoma or other malignancy microscopically, and vice versa. Diagnostic difficulty may arise due to the small and fragmented sample of a core biopsy. This review will focus on the pertinent clinical, radiographic, and histopathologic features of the more commonly encountered inflammatory lesions of the breast that can be characterized in a core biopsy sample. These include fat necrosis, mammary duct ectasia, granulomatous lobular mastitis, diabetic mastopathy, and abscess. The microscopic differential diagnoses for these lesions when seen in a core biopsy sample will be discussed.
Keyword
MeSH Terms
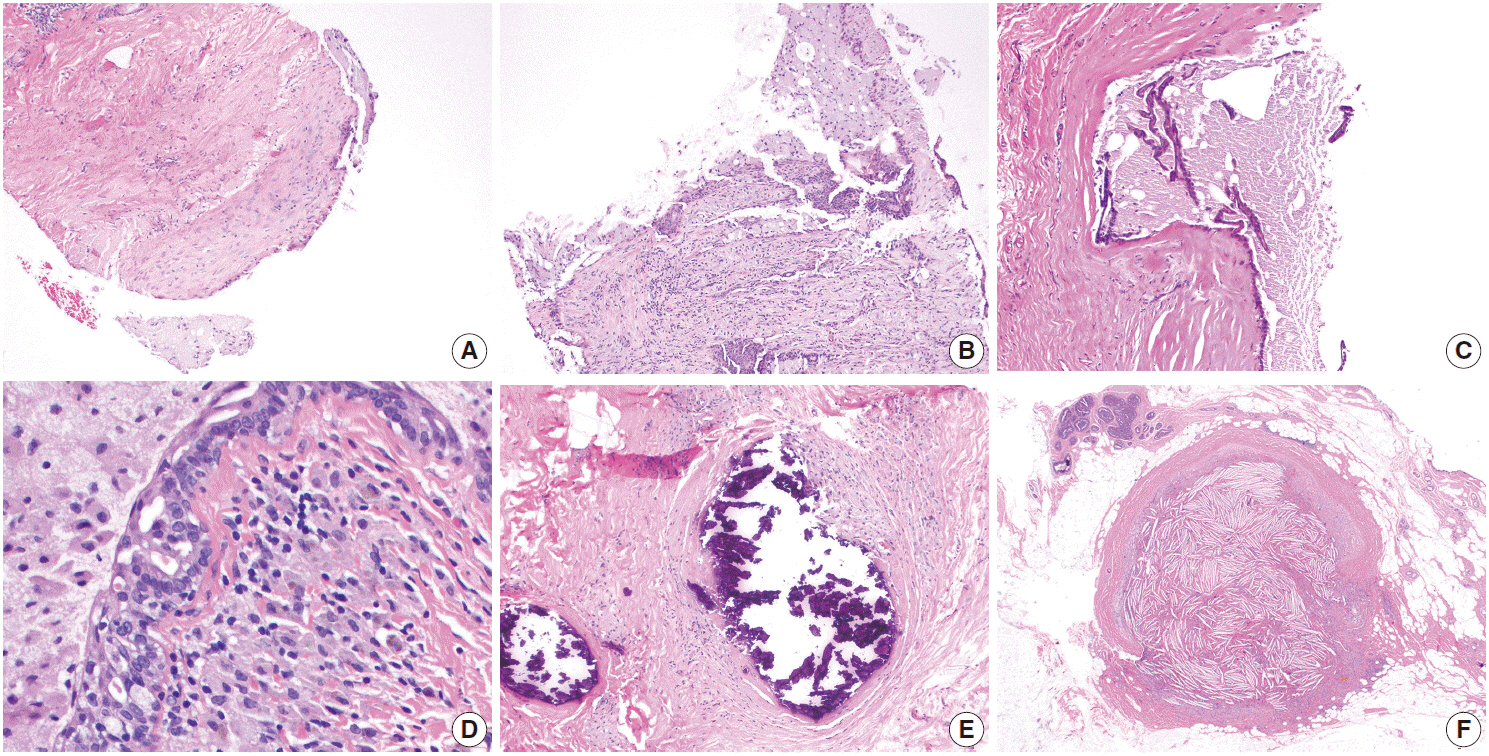
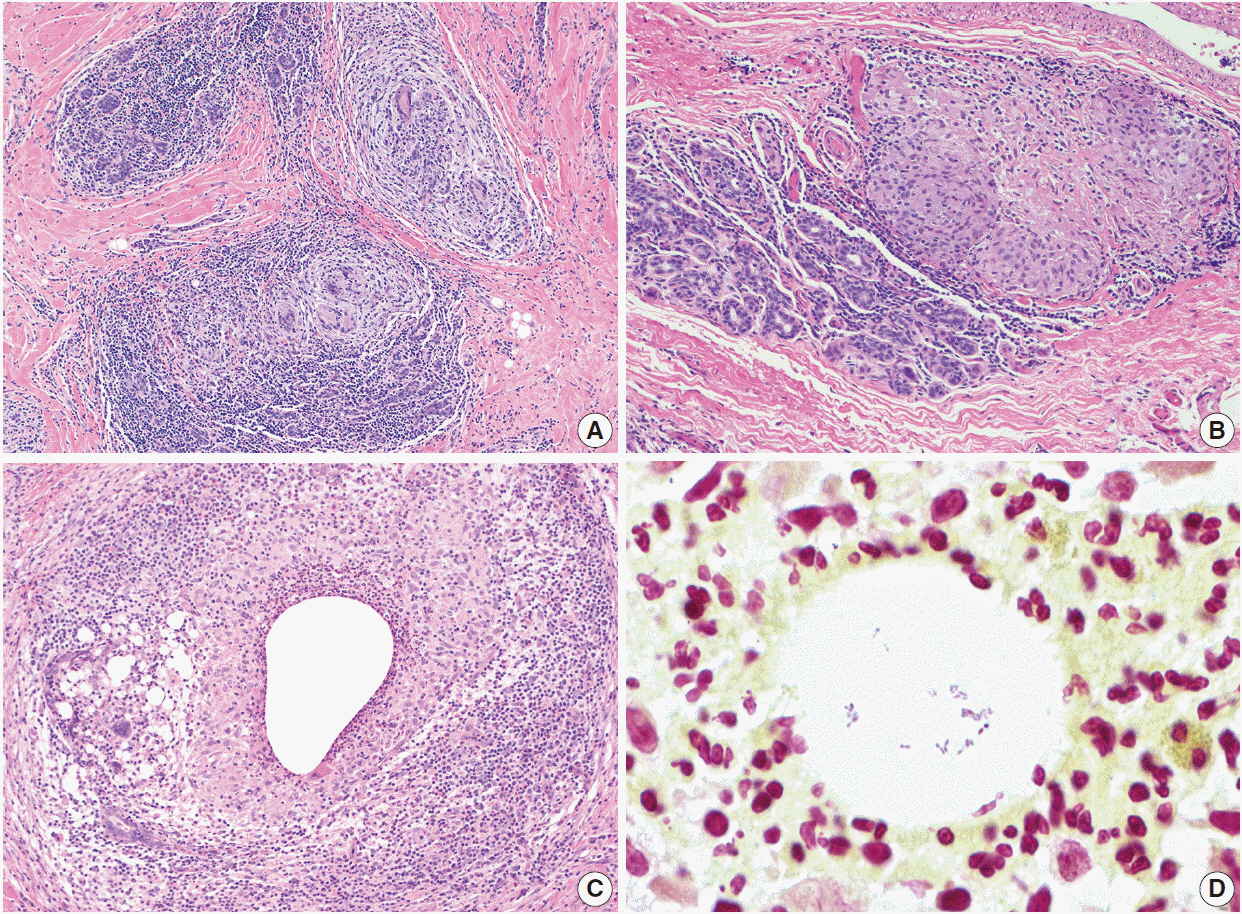
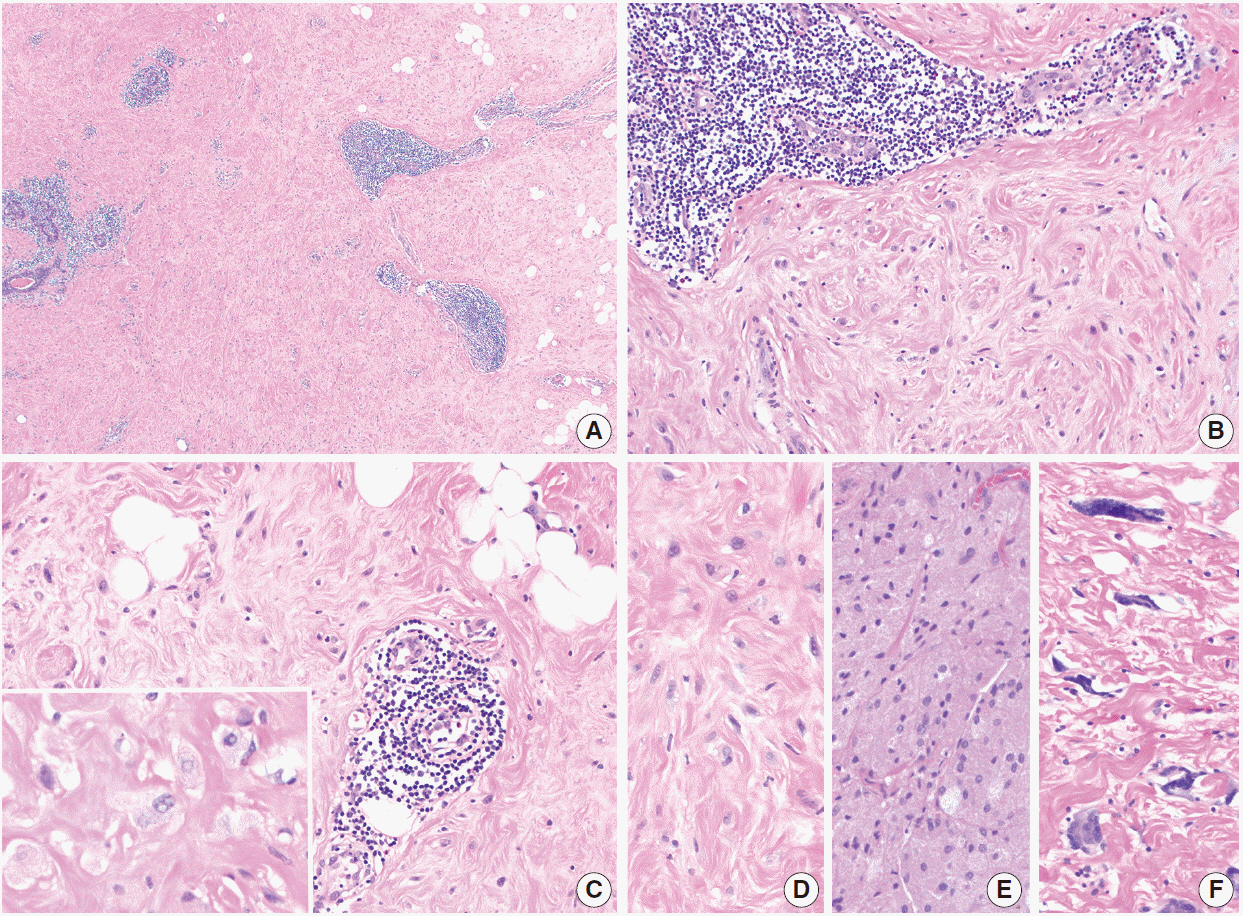
Figure
Reference
-
1. Clarke D, Curtis JL, Martinez A, Fajardo L, Goffinet D. Fat necrosis of the breast simulating recurrent carcinoma after primary radiotherapy in the management of early stage breast carcinoma. Cancer. 1983; 52:442–5.
Article2. Rivera R, Smith-Bronstein V, Villegas-Mendez S, et al. Mammographic findings after intraoperative radiotherapy of the breast. Radiol Res Pract. 2012; 2012:758371.
Article3. Shah C, Badiyan S, Ben Wilkinson J, et al. Treatment efficacy with accelerated partial breast irradiation (APBI): final analysis of the American Society of Breast Surgeons MammoSite((R)) breast brachytherapy registry trial. Ann Surg Oncol. 2013; 20:3279–85.4. Paryani NN, Vallow L, Magalhaes W, et al. The incidence of fat necrosis in balloon-based breast brachytherapy. J Contemp Brachytherapy. 2015; 7:29–34.
Article5. Aqel NM, Howard A, Collier DS. Fat necrosis of the breast: a cytological and clinical study. Breast. 2001; 10:342–5.
Article6. Hogge JP, Robinson RE, Magnant CM, Zuurbier RA. The mammographic spectrum of fat necrosis of the breast. Radiographics. 1995; 15:1347–56.
Article7. Taboada JL, Stephens TW, Krishnamurthy S, Brandt KR, Whitman GJ. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009; 192:815–25.
Article8. Bilgen IG, Ustun EE, Memis A. Fat necrosis of the breast: clinical, mammographic and sonographic features. Eur J Radiol. 2001; 39:92–9.
Article9. Atasoy MM, Oren NC, Ilica AT, Güvenç İ, Günal A, Mossa-Basha M. Sonography of fat necrosis of the breast: correlation with mammography and MR imaging. J Clin Ultrasound. 2013; 41:415–23.
Article10. Soo MS, Kornguth PJ, Hertzberg BS. Fat necrosis in the breast: sonographic features. Radiology. 1998; 206:261–9.
Article11. Morkowski JJ, Nguyen CV, Lin P, et al. Rosai-Dorfman disease confined to the breast. Ann Diagn Pathol. 2010; 14:81–7.
Article12. Guo S, Yan Q, Rohr J, Wang Y, Fan L, Wang Z. Erdheim-Chester disease involving the breast: a rare but important differential diagnosis. Hum Pathol. 2015; 46:159–64.13. Rahal RM, de Freitas-Junior R, Paulinelli RR. Risk factors for duct ectasia. Breast J. 2005; 11:262–5.
Article14. Thomas WG, Williamson RC, Davies JD, Webb AJ. The clinical syndrome of mammary duct ectasia. Br J Surg. 1982; 69:423–5.
Article15. Dixon JM. Periductal mastitis/duct ectasia. World J Surg. 1989; 13:715–20.
Article16. Sweeney DJ, Wylie EJ. Mammographic appearances of mammary duct ectasia that mimic carcinoma in a screening programme. Australas Radiol. 1995; 39:18–23.
Article17. An HY, Kim KS, Yu IK, Kim KW, Kim HH. Image presentation. The nipple-areolar complex: a pictorial review of common and uncommon conditions. J Ultrasound Med. 2010; 29:949–62.18. Davies JD. Pigmented periductal cells (ochrocytes) in mammary dysplasias: their nature and significance. J Pathol. 1974; 114:205–16.
Article19. Oran EŞ, Gürdal SÖ, Yankol Y, et al. Management of idiopathic granulomatous mastitis diagnosed by core biopsy: a retrospective multicenter study. Breast J. 2013; 19:411–8.
Article20. Going JJ, Anderson TJ, Wilkinson S, Chetty U. Granulomatous lobular mastitis. J Clin Pathol. 1987; 40:535–40.
Article21. Ocal K, Dag A, Turkmenoglu O, Kara T, Seyit H, Konca K. Granulomatous mastitis: clinical, pathological features, and management. Breast J. 2010; 16:176–82.
Article22. Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009; 193:574–81.23. Pandey TS, Mackinnon JC, Bressler L, Millar A, Marcus EE, Ganschow PS. Idiopathic granulomatous mastitis: a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014; 20:258–66.24. Ozturk M, Mavili E, Kahriman G, Akcan AC, Ozturk F. Granulomatous mastitis: radiological findings. Acta Radiol. 2007; 48:150–5.
Article25. Al-Khawari HA, Al-Manfouhi HA, Madda JP, Kovacs A, Sheikh M, Roberts O. Radiologic features of granulomatous mastitis. Breast J. 2011; 17:645–50.
Article26. Joseph KA, Luu X, Mor A. Granulomatous mastitis: a New York public hospital experience. Ann Surg Oncol. 2014; 21:4159–63.
Article27. Renshaw AA, Derhagopian RP, Gould EW. Cystic neutrophilic granulomatous mastitis: an underappreciated pattern strongly associated with gram-positive bacilli. Am J Clin Pathol. 2011; 136:424–7.28. Taylor GB, Paviour SD, Musaad S, Jones WO, Holland DJ. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. 2003; 35:109–19.
Article29. D’Alfonso T, Moo TA, Arleo E, Cheng E, Antonio L, Hoda S. Cystic neutrophilic granulomatous lobular mastitis: further clinical and pathological characterization of an under-recognized entity based on eleven cases. Mod Pathol. 2015; 28 Suppl 2:40A–41A.30. Jorgensen MB, Nielsen DM. Diagnosis and treatment of granulomatous mastitis. Am J Med. 1992; 93:97–101.
Article31. Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011; 17:661–8.
Article32. Tomaszewski JE, Brooks JS, Hicks D, Livolsi VA. Diabetic mastopathy: a distinctive clinicopathologic entity. Hum Pathol. 1992; 23:780–6.
Article33. Camuto PM, Zetrenne E, Ponn T. Diabetic mastopathy: a report of 5 cases and a review of the literature. Arch Surg. 2000; 135:1190–3.34. Soler NG, Khardori R. Fibrous disease of the breast, thyroiditis, and cheiroarthropathy in type I diabetes mellitus. Lancet. 1984; 1:193–5.
Article35. Ely KA, Tse G, Simpson JF, Clarfeld R, Page DL. Diabetic mastopathy: a clinicopathologic review. Am J Clin Pathol. 2000; 113:541–5.36. Seidman JD, Schnaper LA, Phillips LE. Mastopathy in insulin-requiring diabetes mellitus. Hum Pathol. 1994; 25:819–24.
Article37. Logan WW, Hoffman NY. Diabetic fibrous breast disease. Radiology. 1989; 172:667–70.
Article38. Moschetta M, Telegrafo M, Triggiani V, et al. Diabetic mastopathy: a diagnostic challenge in breast sonography. J Clin Ultrasound. 2015; 43:113–7.
Article39. Chan CL, Ho RS, Shek TW, Kwong A. Diabetic mastopathy. Breast J. 2013; 19:533–8.
Article40. Dorokhova O, Fineberg S, Koenigsberg T, Wang Y. Diabetic mastopathy, a clinicopathological correlation of 34 cases. Pathol Int. 2012; 62:660–4.
Article41. Tuncbilek N, Karakas HM, Okten O. Diabetic fibrous mastopathy: dynamic contrast-enhanced magnetic resonance imaging findings. Breast J. 2004; 10:359–62.
Article42. Wong KT, Tse GM, Yang WT. Ultrasound and MR imaging of diabetic mastopathy. Clin Radiol. 2002; 57:730–5.
Article43. Valdez R, Thorson J, Finn WG, Schnitzer B, Kleer CG. Lymphocytic mastitis and diabetic mastopathy: a molecular, immunophenotypic, and clinicopathologic evaluation of 11 cases. Mod Pathol. 2003; 16:223–8.
Article44. Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014; 202:W390–9.
Article45. Trop I, Dugas A, David J, et al. Breast abscesses: evidence-based algorithms for diagnosis, management, and follow-up. Radiographics. 2011; 31:1683–99.
Article46. Dener C, Inan A. Breast abscesses in lactating women. World J Surg. 2003; 27:130–3.
Article47. Scott-Conner CE, Schorr SJ. The diagnosis and management of breast problems during pregnancy and lactation. Am J Surg. 1995; 170:401–5.
Article48. Dabbas N, Chand M, Pallett A, Royle GT, Sainsbury R. Have the organisms that cause breast abscess changed with time? Implications for appropriate antibiotic usage in primary and secondary care. Breast J. 2010; 16:412–5.49. Habif DV, Perzin KH, Lipton R, Lattes R. Subareolar abscess associated with squamous metaplasia of lactiferous ducts. Am J Surg. 1970; 119:523–6.
Article50. Versluijs-Ossewaarde FN, Roumen RM, Goris RJ. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J. 2005; 11:179–82.
Article51. Zuska JJ, Crile G Jr, Ayres WW. Fistulas of lactifierous ducts. Am J Surg. 1951; 81:312–7.52. Johnson SP, Kaoutzanis C, Schaub GA. Male Zuska’s disease. BMJ Case Rep. 2014; 2014:bcr2013201922.
Article53. Meguid MM, Oler A, Numann PJ, Khan S. Pathogenesis-based treatment of recurring subareolar breast abscesses. Surgery. 1995; 118:775–82.
Article54. Gollapalli V, Liao J, Dudakovic A, Sugg SL, Scott-Conner CE, Weigel RJ. Risk factors for development and recurrence of primary breast abscesses. J Am Coll Surg. 2010; 211:41–8.
Article55. Lo G, Dessauvagie B, Sterrett G, Bourke AG. Squamous metaplasia of lactiferous ducts (SMOLD). Clin Radiol. 2012; 67:e42–6.
Article56. Walker AP, Edmiston CE Jr, Krepel CJ, Condon RE. A prospective study of the microflora of nonpuerperal breast abscess. Arch Surg. 1988; 123:908–11.
Article57. Kasales CJ, Han B, Smith JS Jr, Chetlen AL, Kaneda HJ, Shereef S. Nonpuerperal mastitis and subareolar abscess of the breast. AJR Am J Roentgenol. 2014; 202:W133–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Ultrasound-Guided Automated Core Biopsy of Nonpalpable Breast Lesions
- Pseudoaneurysm of the Breast after Core Needle Biopsy: A Case Report
- Milk Fistula after Core Biopsy in Lactating Breast: A Case Report
- Effectiveness and Limitations of Core Needle Biopsy in the Diagnosis of Thyroid Nodules: Review of Current Literature
- Screening and Diagnosis for Breast Cancers