Immune Netw.
2013 Dec;13(6):240-248. 10.4110/in.2013.13.6.240.
Increased Lymphocyte Infiltration in Rheumatoid Arthritis Is Correlated with an Increase in LTi-like Cells in Synovial Fluid
- Affiliations
-
- 1Department of Bioinformatics and Life Science, Soongsil University, Seoul 156-743, Korea. kimmy@ssu.ac.kr
- 2Division of Rheumatology, Department of Internal Medicine, Korea University Guro Hospital, Seoul 152-703, Korea.
- 3Department of Otorhinolaryngology, Human Barrier Research Institute, Yonsei University College of Medicine, Seoul 135-720, Korea.
- KMID: 2150784
- DOI: http://doi.org/10.4110/in.2013.13.6.240
Abstract
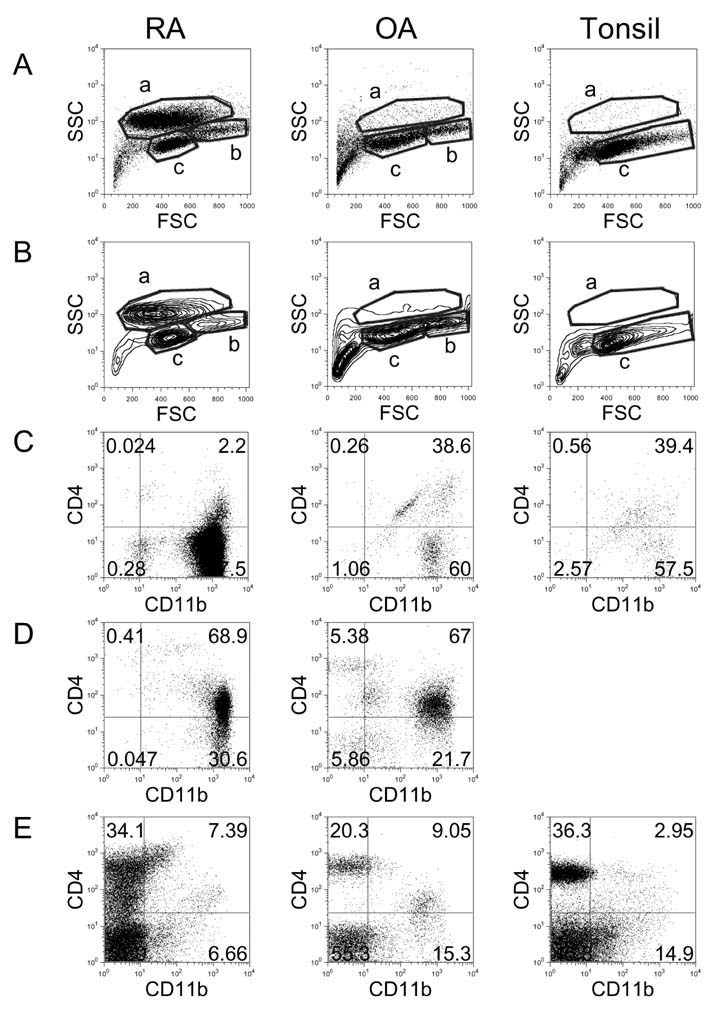
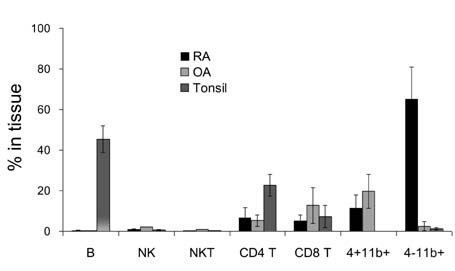
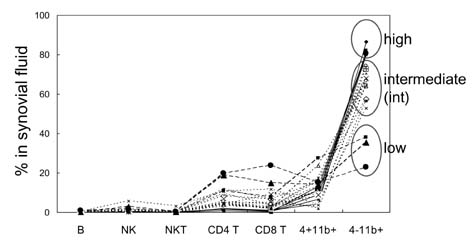
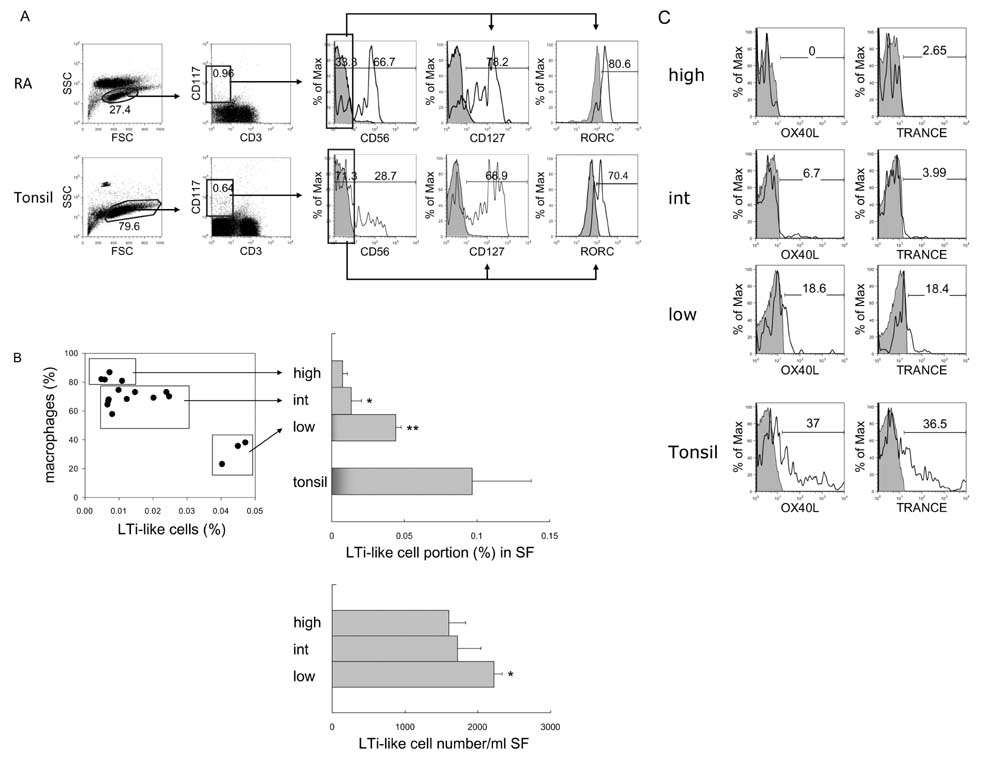
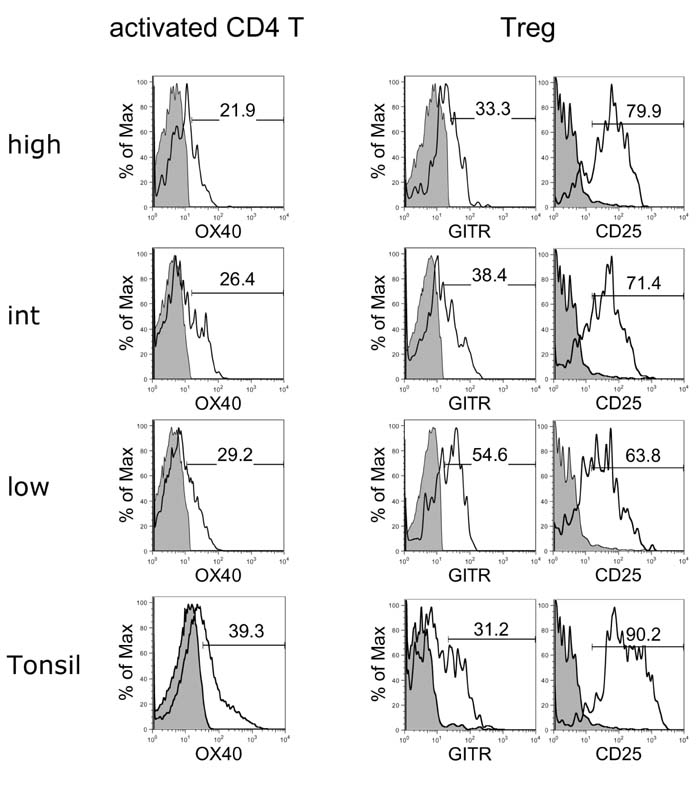
- In this study, we compared the immune cell populations in rheumatoid arthritis (RA) synovial fluid, which shows lymphoid tissue-like structure, with those in tonsils, which are normal secondary lymphoid tissues. Firstly, we found that CD4-CD11b+ macrophages were the major population in RA synovial fluid and that B cells were the major population in tonsils. In addition, synovial fluid from patients with osteoarthritis, which is a degenerative joint disease, contained CD4+CD11b+ monocytes as the major immune cell population. Secondly, we categorized three groups based on the proportion of macrophages found in RA synovial fluid: (1) the macrophage-high group, which contained more than 80% macrophages; (2) the macrophage-intermediate group, which contained between 40% and 80% macrophages; and (3) the macrophage-low group, which contained less than 40% macrophages. In the macrophage-low group, more lymphoid tissue inducer (LTi)-like cells were detected, and the expression of OX40L and TRANCE in these cells was higher than that in the other groups. In addition, in this group, the suppressive function of regulatory T cells was downregulated. Finally, CXCL13 expression was higher in RA synovial fluid than in tonsils, but CCL21 expression was comparable in synovial fluid from all groups and in tonsils. These data demonstrate that increased lymphocyte infiltration in RA synovial fluid is correlated with an increase in LTi-like cells and the elevation of the chemokine expression.
MeSH Terms
Figure
Reference
-
1. Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994; 53:39–44.
Article2. Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000; 2:189–202.3. Cutolo M, Sulli A, Barone A, Seriolo B, Accardo S. Macrophages, synovial tissue and rheumatoid arthritis. Clin Exp Rheumatol. 1993; 11:331–339.4. Young CL, Adamson TC 3rd, Vaughan JH, Fox RI. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984; 27:32–39.
Article5. Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996; 93:221–225.
Article6. Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O'Fallon WM, Goronzy JJ, Weyand CM. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001; 167:1072–1080.
Article7. Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004; 21:655–667.
Article8. Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL, Mebius RE, van der Pouw Kraan TC. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 2007; 56:2492–2502.
Article9. Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999; 189:403–412.
Article10. Wengner AM, Hopken UE, Petrow PK, Hartmann S, Schurigt U, Brauer R, Lipp M. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 2007; 56:3271–3283.
Article11. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007; 7:429–442.
Article12. Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE, Yoshikawa H, Ochi T. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001; 166:650–655.
Article13. Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM 2nd, Shahrara S. Characterization of interleukin-7 and interleukin-7 receptor in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2011; 63:2884–2893.
Article14. Matsui T, Akahoshi T, Namai R, Hashimoto A, Kurihara Y, Rana M, Nishimura A, Endo H, Kitasato H, Kawai S, Takagishi K, Kondo H. Selective recruitment of CCR6-expressing cells by increased production of MIP-3 alpha in rheumatoid arthritis. Clin Exp Immunol. 2001; 125:155–161.
Article15. Bruhl H, Mack M, Niedermeier M, Lochbaum D, Schölmerich J, Straub RH. Functional expression of the chemokine receptor CCR7 on fibroblast-like synoviocytes. Rheumatology (Oxford). 2008; 47:1771–1774.
Article16. Calmon-Hamaty F, Combe B, Hahne M, Morel J. Lymphotoxin alpha stimulates proliferation and pro-inflammatory cytokine secretion of rheumatoid arthritis synovial fibroblasts. Cytokine. 2011; 53:207–214.
Article17. Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997; 7:493–504.
Article18. Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003; 18:643–654.
Article19. Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004; 5:64–73.
Article20. Kim MY, McConnell FM, Gaspal FM, White A, Glanville SH, Bekiaris V, Walker LS, Caamano J, Jenkinson E, Anderson G, Lane PJ. Function of CD4+CD3- cells in relation to B- and T-zone stroma in spleen. Blood. 2007; 109:1602–1610.
Article21. Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007; 26:643–654.
Article22. Kim MY, Rossi S, Withers D, McConnell F, Toellner KM, Gaspal F, Jenkinson E, Anderson G, Lane PJ. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008; 124:166–174.
Article23. Kim S, Han S, Withers DR, Gaspal F, Bae J, Baik S, Shin HC, Kim KS, Bekiaris V, Anderson G, Lane P, Kim MY. CD117(+) CD3(-) CD56(-) OX40Lhigh cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur J Immunol. 2011; 41:1563–1572.
Article24. Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009; 10:66–74.
Article25. Kim MY, Kim KS, McConnell F, Lane P. Lymphoid tissue inducer cells: architects of CD4 immune responses in mice and men. Clin Exp Immunol. 2009; 157:20–26.
Article26. Withers DR, Gaspal FM, Bekiaris V, McConnell FM, Kim M, Anderson G, Lane PJ. OX40 and CD30 signals in CD4+ T-cell effector and memory function: a distinct role for lymphoid tissue inducer cells in maintaining CD4+ T-cell memory but not effector function. Immunol Rev. 2011; 244:134–148.
Article27. Radin EL, Paul IL, Rose RM. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet. 1972; 1:519–522.
Article28. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998; 47:487–504.29. Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003; 197:1191–1198.
Article30. Ohl L, Henning G, Krautwald S, Lipp M, Hardtke S, Bernhardt G, Pabst O, Forster R. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003; 197:1199–1204.
Article31. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–324.
Article32. Wells DA, Benesch M, Loken MR, Vallejo C, Myerson D, Leisenring WM, Deeg HJ. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003; 102:394–403.
Article33. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002; 3:135–142.
Article34. Thurlings RM, Wijbrandts CA, Mebius RE, Cantaert T, Dinant HJ, van der Pouw-Kraan TC, Verweij CL, Baeten D, Tak PP. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum. 2008; 58:1582–1589.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on LD Isoenzyme Activity in Synovial Fluid of Joint Diseases
- Synovial Fluid Adenosine Deaminse Activity in the Patients of Rheumatoid Arthritis, Osteoarthritis, Ankylosing Spondylitis, and Gouty Arthritis
- Ragocytes in Synovial Fluid
- Study of Ferritin Concentration in Synovial Fluid and Serum of Rheumatoid Arthritis
- Clinical significance of the immunological tests in rheumatoid arthritis