Immune Netw.
2012 Dec;12(6):247-252. 10.4110/in.2012.12.6.247.
Inhibition of Human Pancreatic Tumor Growth by Cytokine-Induced Killer Cells in Nude Mouse Xenograft Model
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea. shan@chungbuk.ac.kr
- KMID: 2150756
- DOI: http://doi.org/10.4110/in.2012.12.6.247
Abstract
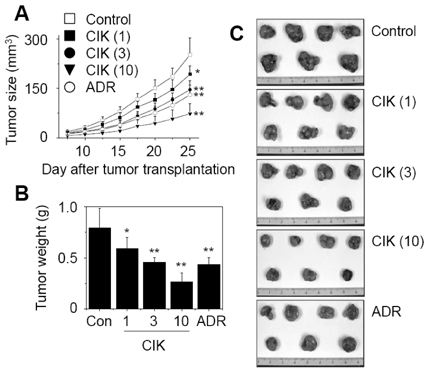
- Pancreatic cancer is the fourth commonest cause of cancer-related deaths in the world. However, no adequate therapy for pancreatic cancer has yet been found. In this study, the antitumor activity of cytokine-induced killer (CIK) cells against the human pancreatic cancer was evaluated in vitro and in vivo. Human peripheral blood mononuclear cells were cultured with IL-2-containing medium in anti-CD3 for 14 days. The resulting populations of CIK cells comprised 94% CD3+, 4% CD3-CD56+, 41% CD3+CD56+, 11% CD4+, and 73% CD8+. This heterogeneous cell population was called cytokine-induced killer (CIK) cells. At an effector-target cell ratio of 100:1, CIK cells destroyed 51% of AsPC-1 human pancreatic cancer cells, as measured by the 51Cr-release assay. In addition, CIK cells at doses of 3 and 10 million cells per mouse inhibited 42% and 70% of AsPC-1 tumor growth in nude mouse xenograft assays, respectively. This study suggests that CIK cells may be used as an adoptive immunotherapy for pancreatic cancer patients.
MeSH Terms
Figure
Reference
-
1. Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford). 2008. 10:58–62.
Article2. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010. 60:277–300.
Article3. Koido S, Homma S, Takahara A, Namiki Y, Tsukinaga S, Mitobe J, Odahara S, Yukawa T, Matsudaira H, Nagatsuma K, Uchiyama K, Satoh K, Ito M, Komita H, Arakawa H, Ohkusa T, Gong J, Tajiri H. Current immunotherapeutic approaches in pancreatic cancer. Clin Dev Immunol. 2011. 2011:267539.
Article4. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997. 15:2403–2413.
Article5. Weng D, Song B, Durfee J, Sugiyama V, Wu Z, Koido S, Calderwood SK, Gong J. Induction of cytotoxic T lymphocytes against ovarian cancer-initiating cells. Int J Cancer. 2011. 129:1990–2001.
Article6. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW. European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004. 350:1200–1210.
Article7. Kim HM, Kang JS, Lim J, Kim JY, Kim YJ, Lee SJ, Song S, Hong JT, Kim Y, Han SB. Antitumor activity of cytokine-induced killer cells in nude mouse xenograft model. Arch Pharm Res. 2009. 32:781–787.
Article8. Franceschetti M, Pievani A, Borleri G, Vago L, Fleischhauer K, Golay J, Introna M. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol. 2009. 37:616–628.e2.9. Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991. 174:139–149.
Article10. Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993. 21:1673–1679.11. Kim HM, Lim J, Park SK, Kang JS, Lee K, Lee CW, Lee KH, Yun MJ, Yang KH, Han G, Kwon SW, Kim Y, Han SB. Antitumor activity of cytokine-induced killer cells against human lung cancer. Int Immunopharmacol. 2007. 7:1802–1807.
Article12. Kim HM, Lim J, Yoon YD, Ahn JM, Kang JS, Lee K, Park SK, Jeong YJ, Kim JM, Han G, Yang KH, Kim YJ, Kim Y, Han SB. Anti-tumor activity of ex vivo expanded cytokine-induced killer cells against human hepatocellular carcinoma. Int Immunopharmacol. 2007. 7:1793–1801.
Article13. Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000. 356:802–807.
Article14. Kornacker M, Moldenhauer G, Herbst M, Weilguni E, Tita-Nwa F, Harter C, Hensel M, Ho AD. Cytokine-induced killer cells against autologous CLL: direct cytotoxic effects and induction of immune accessory molecules by interferon-gamma. Int J Cancer. 2006. 119:1377–1382.
Article15. Yang XJ, Huang JA, Lei W, Zhu YB, Zhang XG. Antitumor effects of cocultured dendritic cells and cytokine-induced killer cells on lung cancer in vitro and in vivo. Ai Zheng. 2006. 25:1329–1333.16. Kim HM, Kang JS, Lim J, Park SK, Lee K, Yoon YD, Lee CW, Lee KH, Han G, Yang KH, Kim YJ, Kim Y, Han SB. Inhibition of human ovarian tumor growth by cytokine-induced killer cells. Arch Pharm Res. 2007. 30:1464–1470.
Article17. Wang W, Epler J, Salazar LG, Riddell SR. Recognition of breast cancer cells by CD8+ cytotoxic T-cell clones specific for NY-BR-1. Cancer Res. 2006. 66:6826–6833.
Article18. Sun S, Li XM, Li XD, Yang WS. Studies on inducing apoptosis effects and mechanism of CIK cells for MGC-803 gastric cancer cell lines. Cancer Biother Radiopharm. 2005. 20:173–180.
Article19. Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004. 103:3065–3072.
Article20. Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID, Yang KH, Kim HM. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology. 1999. 41:157–164.
Article21. Kim JY, Yoon YD, Ahn JM, Kang JS, Park SK, Lee K, Song KB, Kim HM, Han SB. Angelan isolated from Angelica gigas Nakai induces dendritic cell maturation through toll-like receptor 4. Int Immunopharmacol. 2007. 7:78–87.
Article22. Kalinski P, Nakamura Y, Watchmaker P, Giermasz A, Muthuswamy R, Mailliard RB. Helper roles of NK and CD8+ T cells in the induction of tumor immunity. Polarized dendritic cells as cancer vaccines. Immunol Res. 2006. 36:137–146.
Article23. Takashima K, Fujiwara H, Inada S, Atsuji K, Araki Y, Kubota T, Yamagishi H. Tracking of green fluorescent protein (GFP)-labeled LAK cells in mice carrying B16 melanoma metastases. Anticancer Res. 2006. 26:3327–3332.24. Raja Gabaglia C, Diaz de Durana Y, Graham FL, Gauldie J, Sercarz EE, Braciak TA. Attenuation of the glucocorticoid response during Ad5IL-12 adenovirus vector treatment enhances natural killer cell-mediated killing of MHC class I-negative LNCaP prostate tumors. Cancer Res. 2007. 67:2290–2297.
Article25. Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006. 311:1780–1784.
Article26. Grabert RC, Cousens LP, Smith JA, Olson S, Gall J, Young WB, Davol PA, Lum LG. Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res. 2006. 12:569–576.
Article27. Alvarnas JC, Linn YC, Hope EG, Negrin RS. Expansion of cytotoxic CD3+ CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2001. 7:216–222.
Article28. Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994. 153:1687–1696.29. Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011. 118:3301–3310.
Article30. Kim HM, Lim J, Kang JS, Park SK, Lee K, Kim JY, Kim YJ, Hong JT, Kim Y, Han SB. Inhibition of human cervical carcinoma growth by cytokine-induced killer cells in nude mouse xenograft model. Int Immunopharmacol. 2009. 9:375–380.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-tumor activity of tumor necrosis factor alone and combination with VP-16 on renal cell carcinoma in a nude mice xenograft model
- Retrospective growth kinetics and radiosensitivity analysis of various human xenograft models
- An Atopic Nude Mouse Model of Oral Cancer Cell Line
- Adoptive Cell Therapy of Melanoma with Cytokine-induced Killer Cells
- An orthotopic nude mouse model of tongue carcinoma