Immune Netw.
2010 Jun;10(3):85-91. 10.4110/in.2010.10.3.85.
The Impact of Nanomaterials in Immune System
- Affiliations
-
- 1Department of Microbiology, College of Medicine and Nanomedical National Core Research Center, Yonsei University, Seoul, Korea. inhong@yuhs.ac
- KMID: 2150664
- DOI: http://doi.org/10.4110/in.2010.10.3.85
Abstract
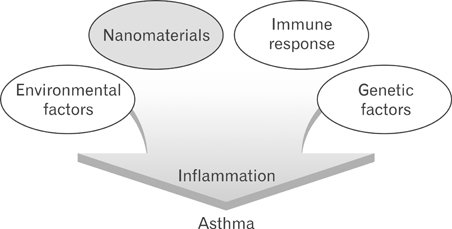
- As a nanotechnology has been actively applied to the overall areas of scientific fields, it is necessary to understand the characteristic features, physical behaviors and the potential effects of exposure to nanomaterials and their toxicity. In this article we review the immunological influences induced by several nanomaterials and emphasize establishment of the animal models to estimate the impact of these nanomaterials on development of immunological diseases.
MeSH Terms
Figure
Cited by 2 articles
-
Phagocytosis and Endocytosis of Silver Nanoparticles Induce Interleukin-8 Production in Human Macrophages
Seungjae Kim, In-Hong Choi
Yonsei Med J. 2012;53(3):654-657. doi: 10.3349/ymj.2012.53.3.654.Cytotoxic Potential of Silver Nanoparticles
Tianlu Zhang, Liming Wang, Qiang Chen, Chunying Chen
Yonsei Med J. 2014;55(2):283-291. doi: 10.3349/ymj.2014.55.2.283.
Reference
-
1. Dreher KL. Health and environmental impact of nanotechnology: toxicological assessment of manufactured nanoparticles. Toxicol Sci. 2004. 77:3–5.
Article2. Borm PJ, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R, Wood S. Research strategies for safety evaluation of nanomaterials, Part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol Sci. 2006. 90:23–32.
Article3. Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, Oberdörster G. Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J Toxicol Environ Health A. 2010. 73:445–461.
Article4. Rivière G. European and international standardisation progress in the field of engineered nanoparticles. Inhal Toxicol. 2009. 21:Suppl 1. 2–7.
Article5. Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Targeting. 2004. 12:635–641.
Article6. Bergamaschi E, Bussolati O, Magrini A, Bottini M, Migliore L, Bellucci S, Iavicoli I, Bergamaschi A. Nanomaterials and lung toxicity: interactions with airways cells and relevance for occupational health risk assessment. Int J Immunopathol Pharmacol. 2006. 19:4 Suppl. 3–10.7. Jou MJ. Pathophysiological and pharmacological implications of mitochondria-targeted reactive oxygen species generation in astrocytes. Adv Drug Deliv Rev. 2008. 60:1512–1526.
Article8. Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annu Rev Public Health. 2009. 30:137–150.
Article9. Møller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010. 44:1–46.
Article10. Girod CE, King TE Jr. COPD: a dust-induced disease? Chest. 2005. 128:3055–3064.11. Biswas P, Wu CY. Nanoparticles and the environment. J Ai Waste Manage Assoc. 2005. 55:708–746.
Article12. Duffin R, Mills NL, Donaldson K. Nanoparticles-a thoracic toxicology perspective. Yonsei Med J. 2007. 48:561–572.
Article13. Alley D, Langley-Turnbaugh S, Gordon N, Wise J, Van Epps G, Jalbert A. The effect of PM10 on human lung fibroblasts. Toxicol Ind Health. 2009. 25:111–120.
Article14. Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010. 118:284–290.
Article15. Nogueira JB. Air pollution and cardiovascular disease. Rev Port Cardiol. 2009. 28:715–733.16. Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010. 42:133–139.17. Rinaudo C, Croce A, Musa M, Fornero E, Allegrina M, Trivero P, Bellis D, Sferch D, Toffalorio F, Veronesi G, Pelosi G. Study of inorganic particles, fibers, and asbestos bodies by variable pressure scanning electron microscopy with annexed energy dispersive spectroscopy and micro-Raman spectroscopy in thin sections of lung and pleural plaque. Appl Spectrosc. 2010. 64:571–577.
Article18. Eom HJ, Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol In Vitro. 2009. 23:1326–1332.
Article19. Eom HJ, Choi J. Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol Lett. 2009. 187:77–83.
Article20. Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, Sadakane K, Yoshikawa T. Effects of nano particles on antigen-related airway inflammation in mice. Respir Res. 2005. 6:106.
Article21. Alberg T, Cassee FR, Groeng EC, Dybing E, Løvik M. Fine ambient particles from various sites in europe exerted a greater IgE adjuvant effect than coarse ambient particles in a mouse model. J Toxicol Environ Health A. 2009. 72:1–13.
Article22. Park EJ, Yoon J, Choi K, Yi J, Park K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. 2009. 260:37–46.
Article23. Liu Y, Jiao F, Qiu Y, Li W, Lao F, Zhou G, Sun B, Xing G, Dong J, Zhao Y, Chai Z, Chen C. The effect of Gd@C82(OH)22 nanoparticles on the release of Th1/Th2 cytokines and induction of TNF-alpha mediated cellular immunity. Biomaterials. 2009. 30:3934–3945.
Article24. Arantes-Costa FM, Lopes FD, Toledo AC, Magliarelli-Filho PA, Moriya HT, Carvalho-Oliveira R, Mauad T, Saldiva PH, Martins MA. Effects of residual oil fly ash (ROFA) in mice with chronic allergic pulmonary inflammation. Toxicol Pathol. 2008. 36:680–686.
Article25. Niwa Y, Hiura Y, Sawamura H, Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circ J. 2008. 72:144–149.
Article26. Koike E, Takano H, Inoue KI, Yanagisawa R, Sakurai M, Aoyagi H, Shinohara R, Kobayashi T. Pulmonary exposure to carbon black nanoparticles increases the number of antigen-presenting cells in murine lung. Int J Immunopathol Pharmacol. 2008. 21:35–42.
Article27. Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, Liu Z, Sun X, Dai H, Gambhir SS. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008. 3:216–221.
Article28. Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi J, Kisin ER, Murray AR, Franks J, Stolz D, Gou P, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010. 5:354–359.
Article29. Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, Chen HW, Yang PC. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008. 8:437–445.
Article30. Herzog E, Byrne HJ, Casey A, Davoren M, Lenz AG, Maier KL, Duschl A, Oostingh GJ. SWCNT suppress inflammatory mediator responses in human lung epithelium in vitro. Toxicol Appl Pharmacol. 2009. 234:378–390.
Article31. Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, Bonner JC. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009. 4:747–751.
Article32. Ye SF, Wu YH, Hou ZQ, Zhang QQ. ROS and NF-kappaB are involved in upregulation of IL-8 in A549 cells exposed to multi-walled carbon nanotubes. Biochem Biophys Res Commun. 2009. 379:643–648.
Article33. Fujita K, Morimoto Y, Ogami A, Myojyo T, Tanaka I, Shimada M, Wang WN, Endoh S, Uchida K, Nakazato T, Yamamoto K, Fukui H, Horie M, Yoshida Y, Iwahashi H, Nakanishi J. Gene expression profiles in rat lung after inhalation exposure to C60 fullerene particles. Toxicology. 2009. 258:47–55.
Article34. Müller L, Riediker M, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J R Soc Interface. 2010. 7:Suppl 1. S27–S40.
Article35. Nemmar A, Melghit K, Ali BH. The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Exp Biol Med (Maywood). 2008. 233:610–619.
Article36. Geiser M, Casaulta M, Kupferschmid B, Schulz H, Semmler-Behnke M, Kreyling W. The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am J Respir Cell Mol Biol. 2008. 38:371–376.
Article37. Yanagisawa R, Takano H, Inoue K, Koike E, Kamachi T, Sadakane K, Ichinose T. Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in NC/Nga mice. Exp Biol Med (Maywood). 2009. 234:314–322.
Article38. Morishige T, Yoshioka Y, Tanabe A, Yao X, Tsunoda S, Tsutsumi Y, Mukai Y, Okada N, Nakagawa S. Titanium dioxide induces different levels of IL-1beta production dependent on its particle characteristics through caspase-1 activation mediated by reactive oxygen species and cathepsin B. Biochem Biophys Res Commun. 2010. 392:160–165.
Article39. Cho WS, Kim S, Han BS, Son WC, Jeong J. Comparison of gene expression profiles in mice liver following intravenous injection of 4 and 100 nm-sized PEG-coated gold nanoparticles. Toxicol Lett. 2009. 191:96–102.
Article40. Hutter E, Boridy S, Labrecque S, Lalancette-Hébert M, Kriz J, Winnik FM, Maysinger D. Microglial response to gold nanoparticles. ACS Nano. 2010. 4:2595–2606.
Article41. Brandenberger C, Rothen-Rutishauser B, Mühlfeld C, Schmid O, Ferron GA, Maier KL, Gehr P, Lenz AG. Effects and uptake of gold nanoparticles deposited at the air-liquid interface of a human epithelial airway model. Toxicol Appl Pharmacol. 2010. 242:56–65.
Article42. Park EJ, Kim H, Kim Y, Yi J, Choi K, Park K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology. 2010. PMID: 20540983.
Article43. Zhong CY, Zhou YM, Smith KR, Kennedy IM, Chen CY, Aust AE, Pinkerton KE. Oxidative injury in the lungs of neonatal rats following short-term exposure to ultrafine iron and soot particles. J Toxicol Environ Health A. 2010. 73:837–847.
Article44. Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008. 2:2121–2134.
Article45. Park YH, Kim JN, Jeong SH, Choi JE, Lee SH, Choi BH, Lee JP, Sohn KH, Park KL, Kim MK, Son SW. Assessment of dermal toxicity of nanosilica using cultured keratinocytes, a human skin equivalent model and an in vivo model. Toxicology. 2010. 267:178–181.
Article46. Li X, Hu Y, Jin Z, Jiang H, Wen J. Silica-induced TNF-alpha and TGF-beta1 expression in RAW264.7 cells are dependent on Src-ERK/AP-1 pathways. Toxicol Mech Methos. 2009. 19:51–58.
Article47. Nishimori H, Kondoh M, Isoda K, Tsunoda S, Tsutsumi Y, Yagi K. Silica nanoparticles as hepatotoxicants. Eur J Pharm Biopharm. 2009. 72:496–501.
Article48. Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008. 2:85–96.
Article49. Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008. 38:1404–1413.
Article50. Inoue K, Takano H, Yanagisawa R, Koike E, Shimada A. Size effects of latex nanomaterials on lung inflammation in mice. Toxicol Appl Pharmacol. 2009. 234:68–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Network Receives First Impact Factor of 2.524
- Impacts of the Spaceflight to the Immune System
- Aging and the Immune System: the Impact of Immunosenescence on Viral Infection, Immunity and Vaccine Immunogenicity
- Risk Assessment Principle for Engineered Nanotechnology in Food and Drug
- Interplay between Intestinal Microbiota and Host Immune System