J Korean Soc Radiol.
2016 Jan;74(1):26-36. 10.3348/jksr.2016.74.1.26.
Infusion Sclerotherapy of Microcystic Lymphatic Malformation: Clinico-Radiological Mid-Term Results
- Affiliations
-
- 1Department of Radiology, Department of Surgery, Kyungpook National University Hospital, Daegu, Korea. jonglee@knu.ac.kr
- 2Department of Dermartology, Department of Surgery, Kyungpook National University Hospital, Daegu, Korea.
- 3Department of Plastic Surgery, Department of Surgery, Kyungpook National University Hospital, Daegu, Korea.
- 4Division of Vascular Surgery, Department of Surgery, Kyungpook National University Hospital, Daegu, Korea.
- KMID: 2150460
- DOI: http://doi.org/10.3348/jksr.2016.74.1.26
Abstract
- PURPOSE
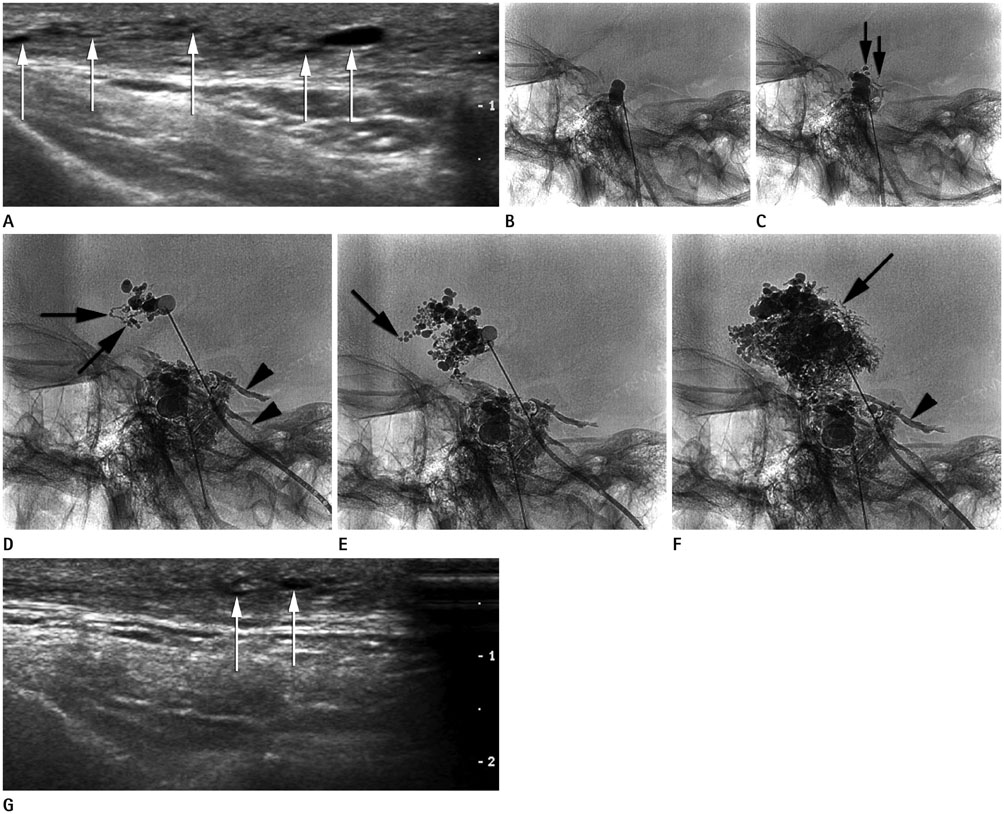
A new sclerotherapy technique by slow mechanical infusion of sclerosant was validated for treatment of microcystic lymphatic malformation (mLM).
MATERIALS AND METHODS
Seventeen consecutive patients with mLM in extremities, cervicofacial area, and trunk were included (21.8 +/- 21.5 years old, male:female = 5:12). All patients diagnosed as mLM were included. A total 4-32 mL 20-38% OK-432 solution was mechanically infused at the rate of 10 mL/hour into the mLM lesions. The treatment effect was estimated clinic-radiologically at the 4-month follow-up. Repeated sclerotherapy followed in the 6th month, if required. The therapeutic effect was evaluated using quantitative ultrasonographic examination including soft tissue thickness, cyst size and number.
RESULTS
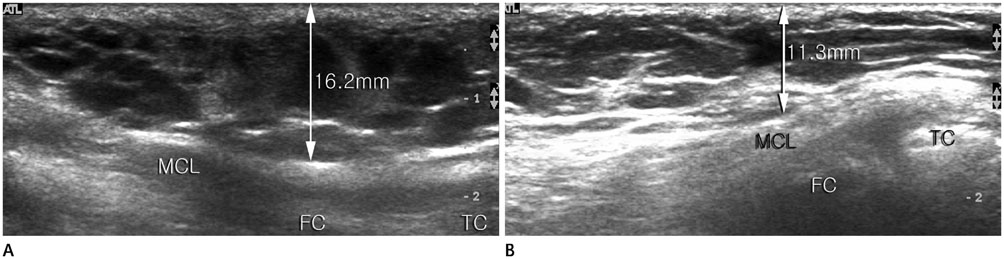
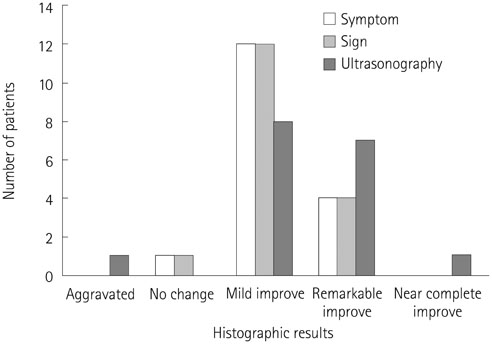
In 17 patients, total 31 infusion sclerotherapy sessions were performed and monitored for 425 +/- 266 days. Fifteen patients (88%) showed improvement in all symptoms, signs, and ultrasonographic findings. In all cases, at least one finding presented improvement. The maximal number of cysts per ultrasonographic window and maximal diameter of the largest cyst decreased by 57 +/- 57% and 51 +/- 67%, respectively (p = 0.102, 0.004). The soft tissue thickness decreased by 18 +/- 15% (p < 0.01). No significant complications such as distal lymphedema or skin necrosis occurred.
CONCLUSION
Infusion sclerotherapy is a safe and effective treatment technique for microcystic LM, with improved outcome.
MeSH Terms
Figure
Reference
-
1. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982; 69:412–422.2. Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol. 1997; 24:701–710.3. Giguère CM, Bauman NM, Sato Y, Burke DK, Greinwald JH, Pransky S, et al. Treatment of lymphangiomas with OK-432 (Picibanil) sclerotherapy: a prospective multi-institutional trial. Arch Otolaryngol Head Neck Surg. 2002; 128:1137–1144.4. Bond J, Basheer MH, Gordon D. Lymphangioma circumscriptum: pitfalls and problems in definitive management. Dermatol Surg. 2008; 34:271–275.5. Renton JP, Smith RJ. Current treatment paradigms in the management of lymphatic malformations. Laryngoscope. 2011; 121:56–59.6. Smith MC, Zimmerman MB, Burke DK, Bauman NM, Sato Y, Smith RJ. OK-432 Collaborative Study Group. Efficacy and safety of OK-432 immunotherapy of lymphatic malformations. Laryngoscope. 2009; 119:107–115.7. Bai Y, Jia J, Huang XX, Alsharif MJ, Zhao JH, Zhao YF. Sclerotherapy of microcystic lymphatic malformations in oral and facial regions. J Oral Maxillofac Surg. 2009; 67:251–256.8. Shiels WE 2nd, Kang DR, Murakami JW, Hogan MJ, Wiet GJ. Percutaneous treatment of lymphatic malformations. Otolaryngol Head Neck Surg. 2009; 141:219–224.9. Zhou Q, Zheng JW, Mai HM, Luo QF, Fan XD, Su LX, et al. Treatment guidelines of lymphatic malformations of the head and neck. Oral Oncol. 2011; 47:1105–1109.10. Cahill AM, Nijs E, Ballah D, Rabinowitz D, Thompson L, Rintoul N, et al. Percutaneous sclerotherapy in neonatal and infant head and neck lymphatic malformations: a single center experience. J Pediatr Surg. 2011; 46:2083–2095.11. Yang Y, Sun M, Ma Q, Cheng X, Ao J, Tian L, et al. Bleomycin A5 sclerotherapy for cervicofacial lymphatic malformations. J Vasc Surg. 2011; 53:150–155.12. Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010; 36:1711–1717.13. Poldervaart MT, Breugem CC, Speleman L, Pasmans S. Treatment of lymphatic malformations with OK-432 (picibanil): review of the literature. J Craniofac Surg. 2009; 20:1159–1162.14. Alomari AI, Karian VE, Lord DJ, Padua HM, Burrows PE. Percutaneous sclerotherapy for lymphatic malformations: a retrospective analysis of patient-evaluated improvement. J Vasc Interv Radiol. 2006; 17:1639–1648.15. Hogeling M, Adams S, Law J, Wargon O. Lymphatic malformations: clinical course and management in 64 cases. Australas J Dermatol. 2011; 52:186–190.16. Rautio R, Keski-Nisula L, Laranne J, Laasonen E. Treatment of lymphangiomas with OK-432 (picibanil). Cardiovasc Intervent Radiol. 2003; 26:31–36.17. Smith RJ. Lymphatic malformations. Lymphat Res Biol. 2004; 2:25–31.18. Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years' experience with lymphangiomas in children. J Pediatr Surg. 1999; 34:1164–1168.19. Mathur NN, Rana I, Bothra R, Dhawan R, Kathuria G, Pradhan T. Bleomycin sclerotherapy in congenital lymphatic and vascular malformations of head and neck. Int J Pediatr Otorhinolaryngol. 2005; 69:75–80.20. Nehra D, Jacobson L, Barnes P, Mallory B, Albanese CT, Sylvester KG. Doxycycline sclerotherapy as primary treatment of head and neck lymphatic malformations in children. J Pediatr Surg. 2008; 43:451–460.21. Herbreteau D, Riche MC, Enjolras O, Khayata M, Lemarchand-Venencie F, Borsik M, et al. Percutaneous embolization with Ethibloc of lymphatic cystic malformations with a review of the experience in 70 patients. Int Angiol. 1993; 12:34–39.22. Park HS, Do YS, Park KB, Kim DI, Kim YW, Kim MJ, et al. Ethanol embolotherapy of hand arteriovenous malformations. J Vasc Surg. 2011; 53:725–731.23. Orlando JL, Caldas JG, Campos HG, Nishinari K, Wolosker N. Outpatient percutaneous treatment of deep venous malformations using pure ethanol at low doses under local anesthesia. Clinics (Sao Paulo). 2010; 65:837–884.24. Yura J, Hashimoto T, Tsuruga N, Shibata K. Bleomycin treatment for cystic hygroma in children. Nihon Geka Hokan. 1977; 46:607–614.25. Levy RL, Chiarillo S. Hyperpyrexia, allergic-type response and death occurring with low-dose bleomycin administration. Oncology. 1980; 37:316–317.26. Sung MW, Chang SO, Choi JH, Kim JY. Bleomycin sclerotherapy in patients with congenital lymphatic malformation in the head and neck. Am J Otolaryngol. 1995; 16:236–241.27. Chaudry G, Guevara CJ, Rialon KL, Kerr C, Mulliken JB, Greene AK, et al. Safety and efficacy of bleomycin sclerotherapy for microcystic lymphatic malformation. Cardiovasc Intervent Radiol. 2014; 37:1476–1481.28. Shergill A, John P, Amaral JG. Doxycycline sclerotherapy in children with lymphatic malformations: outcomes, complications and clinical efficacy. Pediatr Radiol. 2012; 42:1080–1088.29. Burrows PE, Mitri RK, Alomari A, Padua HM, Lord DJ, Sylvia MB, et al. Percutaneous sclerotherapy of lymphatic malformations with doxycycline. Lymphat Res Biol. 2008; 6:209–216.30. Fujino A, Moriya Y, Morikawa Y, Hoshino K, Watanabe T, Shimojima N, et al. A role of cytokines in OK-432 injection therapy for cystic lymphangioma: an approach to the mechanism. J Pediatr Surg. 2003; 38:1806–1809.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiofrequency Ablation of Microcystic Lymphatic Malformation in the Oral Cavity: 2 Case Studies
- A Case of Klippel-Trenaunay Syndrome with Microcystic Lymphatic Malformation on Anus
- Percutaneous Drainage and Povidone-Iodine Sclerotherapy of Cervical Lymphatic Malformation
- Sclerotherapy of cystic lymphangioma
- Clicically improved venous malformation by sclerotherapy