Obstet Gynecol Sci.
2015 May;58(3):232-238. 10.5468/ogs.2015.58.3.232.
Relationship between phospholipase C zeta immunoreactivity and DNA fragmentation and oxidation in human sperm
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. blasto@snubh.org
- 2Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Obstetrics and Gynecology, CHA Gangnam Medical Center, CHA University, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul, Korea.
- KMID: 2148936
- DOI: http://doi.org/10.5468/ogs.2015.58.3.232
Abstract
OBJECTIVE
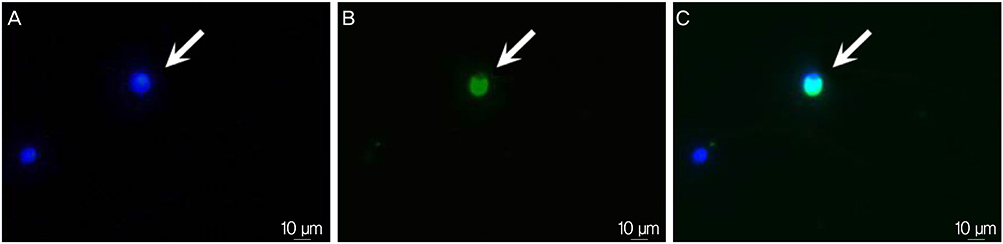
The study aimed to evaluate the feasibility and reproducibility of measuring phospholipase C zeta (PLCzeta) using immunostaining in human sperm and to investigate the relationship between PLCzeta immunoreactivity and DNA fragmentation and oxidation in human sperm.
METHODS
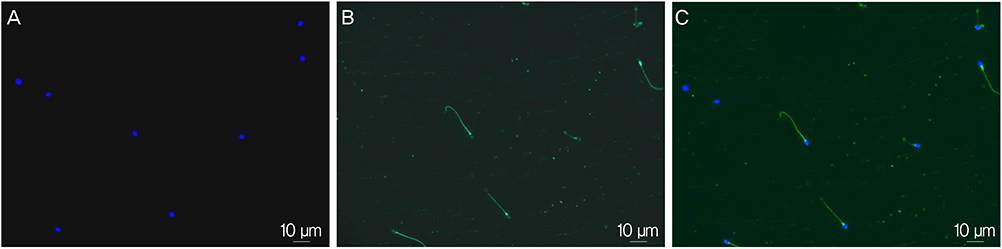
Semen samples were obtained from participants (n=44) and processed by the conventional swim-up method. Sperm concentration, motility, normal form by strict morphology, DNA fragmentation index assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling method and immunofluorescent expression for 8-hydroxy-2'-deoxyguanosine (8-OHdG) and PLCzeta were assessed.
RESULTS
When duplicate PLCzeta tests were performed on two sperm samples from each of the 44 participants, similar results were obtained (74.1+/-9.4% vs. 75.4+/-9.7%). Two measurements of PLCzeta were found to be highly correlated with each other (r=0.759, P<0.001). Immunoreactivity of PLCzeta was not associated with donor's age, sperm concentration, motility, and the percentage of normal form as well as DNA fragmentation index. However, immunoreactivity of PLCzeta showed a significant negative relationship with 8-OHdG immunoreactivity (r=-0.404, P=0.009).
CONCLUSION
Measurement of PLCzeta by immunostaining is feasible and reproducible. Lower expression of PLCzeta in human sperm may be associated with higher sperm DNA oxidation status.
MeSH Terms
Figure
Reference
-
1. Grasa P, Coward K, Young C, Parrington J. The pattern of localization of the putative oocyte activation factor, phospholipase Czeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod. 2008; 23:2513–2522.2. Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod Biomed Online. 2008; 17:662–668.3. Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2010; 94:520–526.4. Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005; 20:2237–2241.5. Garrido N, Martinez-Conejero JA, Jauregui J, Horcajadas JA, Simon C, Remohi J, et al. Microarray analysis in sperm from fertile and infertile men without basic sperm analysis abnormalities reveals a significantly different transcriptome. Fertil Steril. 2009; 91:1307–1310.6. Meseguer M, de los Santos MJ, Simon C, Pellicer A, Remohi J, Garrido N. Effect of sperm glutathione peroxidases 1 and 4 on embryo asymmetry and blastocyst quality in oocyte donation cycles. Fertil Steril. 2006; 86:1376–1385.7. Aghajanpour S, Ghaedi K, Salamian A, Deemeh MR, Tavalaee M, Moshtaghian J, et al. Quantitative expression of phospholipase C zeta, as an index to assess fertilization potential of a semen sample. Hum Reprod. 2011; 26:2950–2956.8. Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011; 18:1005–1013.9. Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011; 57:78–85.10. Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010; 16:3–13.11. Jee BC, Suh CS, Shin MS, Lee HJ, Lee JH, Kim SH. Sperm nuclear DNA fragmentation and chromatin structure in one-day-old ejaculated sperm. Clin Exp Reprod Med. 2011; 38:82–86.12. Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod. 2009; 24:2417–2428.13. Garrido N, Meseguer M, Alvarez J, Simon C, Pellicer A, Remohi J. Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil Steril. 2004; 82:Suppl 3. 1059–1066.14. Morado S, Cetica P, Beconi M, Thompson JG, Dalvit G. Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated bovine oocyte activation. Reproduction. 2013; 145:471–478.15. Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010; 25:2415–2426.16. Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991; 88:11003–11006.17. Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997; 68:519–524.18. Sergerie M, Ouhilal S, Bissonnette F, Brodeur J, Bleau G. Lack of association between smoking and DNA fragmentation in the spermatozoa of normal men. Hum Reprod. 2000; 15:1314–1321.19. Shen HM, Chia SE, Ni ZY, New AL, Lee BL, Ong CN. Detection of oxidative DNA damage in human sperm and the association with cigarette smoking. Reprod Toxicol. 1997; 11:675–680.20. Ji G, Yan L, Liu W, Qu J, Gu A. OGG1 Ser326Cys polymorphism interacts with cigarette smoking to increase oxidative DNA damage in human sperm and the risk of male infertility. Toxicol Lett. 2013; 218:144–149.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlations between abnormalities of morphological details and DNA fragmentation in human sperm
- What should be done for men with sperm DNA fragmentation?
- Phospholipase C zeta: a hidden face of sperm for oocyte activation and early embryonic development
- Transition nuclear protein 1 as a novel biomarker in patients with fertilization failure
- ICSI using fresh and frozen PESA-TESA spermatozoa to examine assisted reproductive outcome retrospectively