Korean J Lab Med.
2006 Feb;26(1):45-51. 10.3343/kjlm.2006.26.1.45.
Experimental Application of Whole Blood Flow Cytometry to HLA Crossmatch for Renal Transplantation
- Affiliations
-
- 1Department of Clinical Pathology, Kyungpook National University School of Medicine, Daegu, Korea. wondi@knu.ac.kr
- KMID: 2143197
- DOI: http://doi.org/10.3343/kjlm.2006.26.1.45
Abstract
-
BACKGROUND: The lymphocytes separated from whole blood are used in HLA flow cytometry crossmatch (FCXM) for renal transplantation. In this study, the methodology of whole blood flow cytometry was applied to FCXM, omitting lymphocyte separation step.
METHODS
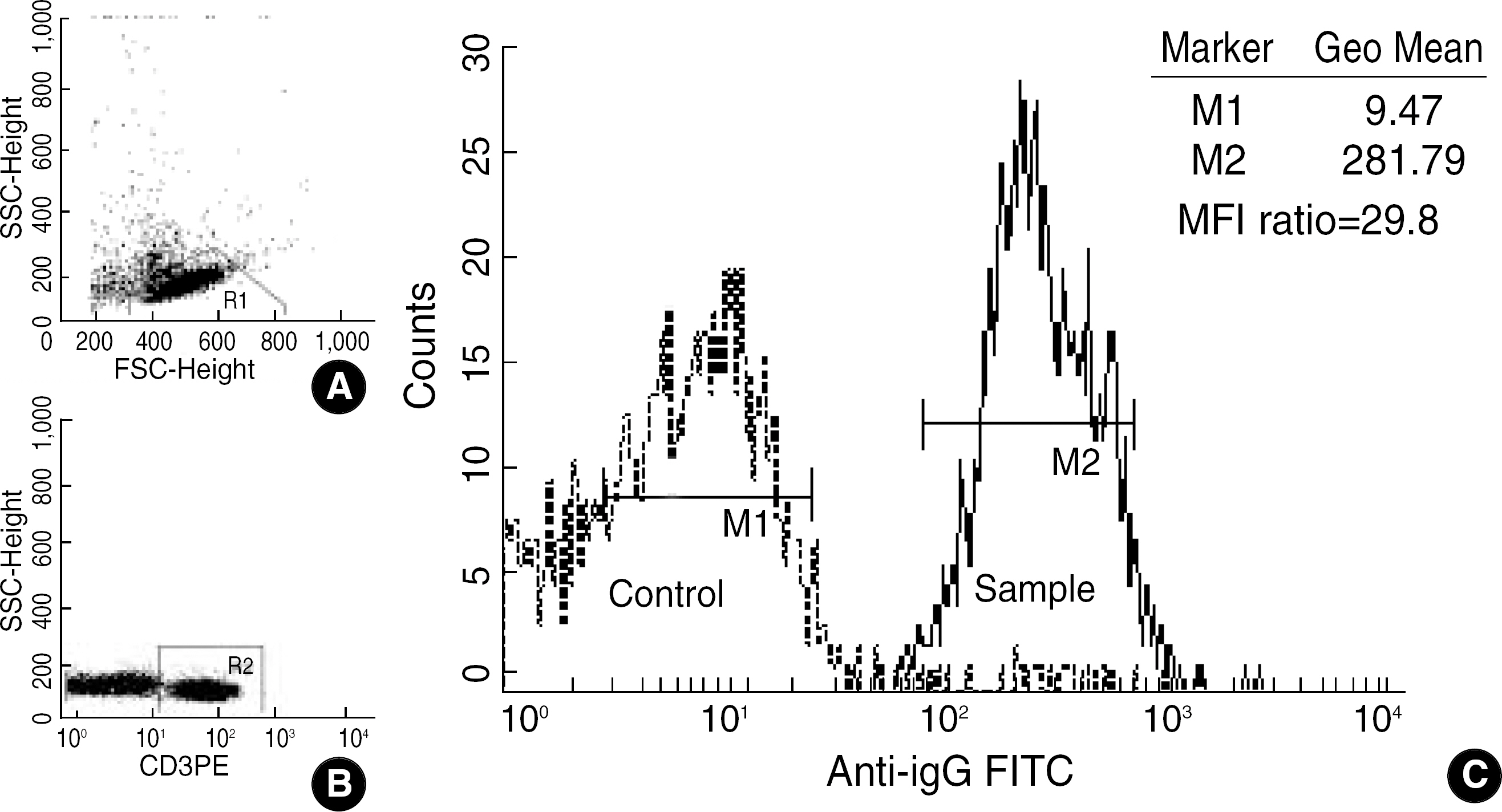
In the 20 cases (including positive 5 cases) of T cell FCXM for renal transplantation, the standard assay using the separated mononuclear cells (MNC) was compared with the two variant assays using whole blood. In the latter assay, the donor whole blood was incubated with the excessive recipient serum. The red cells were lysed (lysed whole blood, LWB). Otherwise, instead of red cell lysis, the signals of T cells among whole blood (WB) were acquired using fluorescence triggering. The sample/negative control mean fluorescence intensity (MFI) ratio was calculated for the interpretation.
RESULTS
The MFI ratio of the 20 cases by MNC, LWB and WB assay were 4.9+/-8.1, 5.4+/-9.7 and 4.8+/-7.8, respectively. Both LWB and WB assay were not significantly different from MNC assay (P= 0.313, 0.831, respectively, paired t-test). The qualitative determinations were concordant in all cases, except for one case which was weakly positive with MFI ratio 2.2 by LWB assay.
CONCLUSIONS
The assays using whole blood were comparable to the standard assay in FCXM for renal transplantation. This study indirectly supports that the variant methods can be used reliably in the case of the MNC preparation erroneously mixed with other blood cells.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Analysis of Positive Flow Cytometric Crossmatch in Organ Transplantation
Yun Soo Lee, Dong Il Won
Lab Med Online. 2011;1(1):43-50. doi: 10.3343/lmo.2011.1.1.7.A Successful Case of a High Anti A/B Antibody Titer ABO Incompatible Kidney Transplantation Patient Who Received a Kidney from a Hepatitis B Carrier
Jin Ho Lee, Han Sae Kim, Dong Yeol Lee, Joon Seok Oh, Yong Hun Sin, Joong Kyung Kim, Jong Hyun Park, Kill Huh, Jong In Park
J Korean Soc Transplant. 2016;30(4):184-189. doi: 10.4285/jkstn.2016.30.4.184.
Reference
-
References
1. 대한진단검사의학회(정도관리분과위원회). 제13차 HLA 검사 신빙도 조사분석결과 2004.2. Hoy T, Garner S, Shenton BK, Bell AE, Lowdell MW, Farrant J, North M, Sewell C. Further clinical applications. Ormerod MG, editor. Flow cytometry Third Edition. New York: Oxford University Press;2000. p. 99–124.3. Robert AB. Flow Cytometry Crossmatching for solid organ transplantation. Darzynkiewicz Z, Robinson JP, editors. Method in cell biology: volume 41 flow cytometry part A. 2nd ed.San Diego: Academic Press;1994. p. 437–47.4. von dem Borne AE, Verheugt FW, Oosterhof F, von Riesz E, de la Riviere AB, Engelfriet CP. A simple immunofluorescence test for the detection of platelet antibodies. Br J Haematol. 1978; 39:195–207.
Article5. Oh WI, Park MH, Han KS. Detection of serum platelet antibodies using microplate platelet suspension immunofluorescence test. Korean J Clin Pathol. 1990; 10:403–10.6. De Caterina M, Grimaldi E, Ungaro B, Fratellanza G, Varriale V, Ciarnelli M, et al. Effect of paraformaldehyde on platelet size and on measurement of surface IgG. Platelets. 2002; 13:207–12.
Article7. National Institute for Biological Standards and Control. Platelet immunofluorescence test. http://www.nibsc.ac.uk/aboutus/platelets.asp?id=28.8. Robinson MS, MacKie IJ, Machin SJ, Harrison P. Two colour analysis of reticulated platelets. Clin Lab Haematol. 2000; 22:211–3.
Article9. Tait JF, Smith C, Wood BL. Measurement of phosphatidylserine exposure in leukocytes and platelets by whole-blood flow cytometry with annexin V. Blood Cells Mol Dis. 1999; 25:271–8.
Article10. Alvarez-Larran A, Jover L, Marin P, Petriz J. A multicolor, no-lyse no-wash assay for the absolute counting of CD34+ cells by flow cytometry. Cytometry. 2002; 50:249–53.
Article11. Li N, Goodall AH, Hjemdahl P. Efficient flow cytometric assay for platelet-leukocyte aggregates in whole blood using fluorescence signal triggering. Cytometry. 1999; 35:154–61.
Article12. Sigma-Aldrich, Inc. Histopaque-1077 package insert. St. Louis: Sigma-Aldrich Inc.;2003.13. Robert AB, Howard MG. Clinical utility of flow cytometry in allogeneic transplantation. Keren FD, McCoy JP, editors. Flow cytometry in clinical diagnosis. 3rd ed.Chicago: ASCP Press;2001. p. 507–41.14. 김유경, 허운보, 원동일, 서장수.유세포분석교차시험에서양성을보인 신장이식 예정 환자에서 혈장교환술을 시행한1예. 대한진단검사의학회 지2004;24(S2):S422.15. Zhang Q, Liang LW, Gjertson DW, Lassman C, Wilkinson AH, Kendrick E, et al. Development of posttransplant antidonor HLA antibodies is associated with acute humoral rejection and early graft dysfunction. Transplantation. 2005; 79:591–8.
Article16. GTI, Inc. Antibody Monitoring System (AMS): HLA Class I/II package insert. Waukesha: GTI, Inc.;2004.17. Wahrmann M, Exner M, Regele H, Derfler K, Kormoczi GF, Lhotta K, et al. Flow cytometry based detection of HLA alloantibody mediated classical complement activation. J Immunol Methods. 2003; 275:149–60.
Article18. Ta M, Scornik JC. Improved flow cytometric detection of donor-specific HLA class II antibodies by heat inactivation. Transplantation. 2002; 73:1611–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Flow Cytometric AHG-CDC for HLA Crossmatch: A Pilot Study
- Plasmapheresis in a Renal Transplant Patient with Positive Crossmatch only Detected by Flow Cytometry
- Results of the HLA Typing Proficiency Survey in Korea, 2000-2002
- A Living Donor Liver Transplantation after Therapeutic Plasmapheresis in a Patient with Positive HLA Crossmatch
- Comparison of Flow Cytometry Crossmatch with Conventional Lymphocytotoxic Crossmatch in Living Donor Renal Transplantation