J Korean Med Assoc.
2009 Jan;52(1):69-77. 10.5124/jkma.2009.52.1.69.
Functional Mapping of Nervous System Using Optical Imaging Techniques
- Affiliations
-
- 1Department of Physiology, Yonsei University College of Medicine, Korea.
- 2Department of Anesthesiology and Pain Medicine, Yonsei University College of Medicine, Korea. sjbai1@yuhs.ac
- KMID: 2137735
- DOI: http://doi.org/10.5124/jkma.2009.52.1.69
Abstract
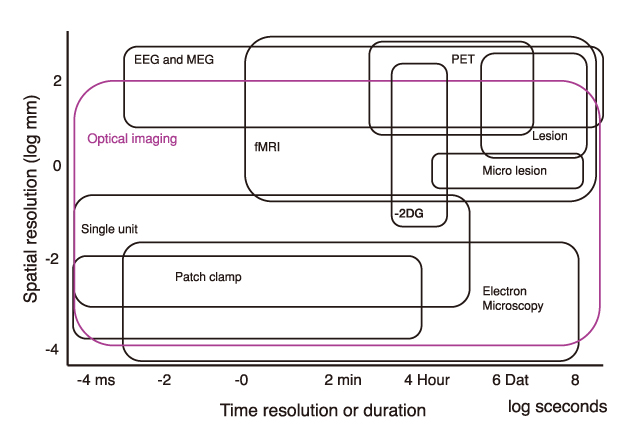
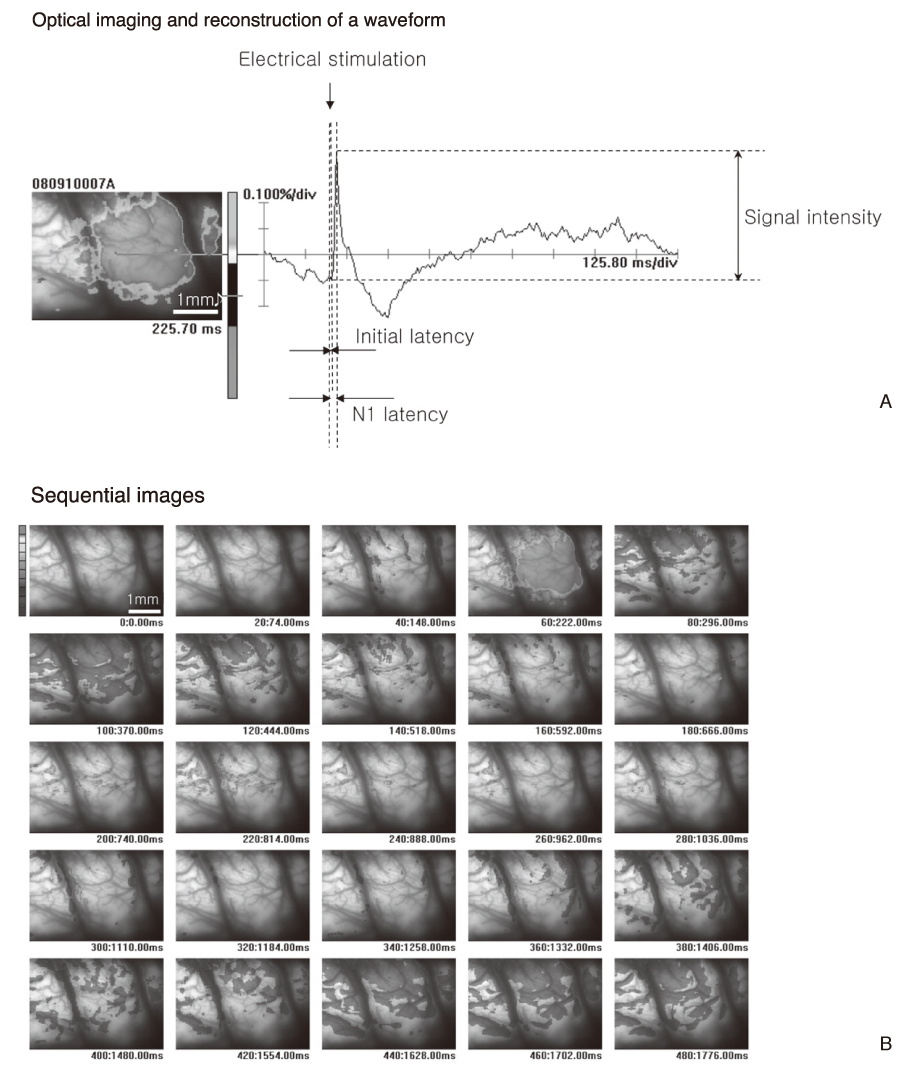
- Functional mapping techniques including functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and magnetoencephalography (MEG) can be used to study the function of the nervous system. Optical imaging is an emerging technique for functional imaging of the nervous tissue. Functional optical imaging can be classified into two major streams; intrinsic signal optical imaging (ISO) and voltage-sensitive dye optical imaging (VDO). ISO is related to hemodynamic changes such as hemoglobin concentration and oxygenation changes, cytochrome oxidation change, and light scattering. On the contrary, VOD measures changes in membrane potentials of neural cells. Therefore, ISO reflects metabolic activity of neurons, while VOD directly reflects neural activity. Recent advances in optical imaging opened the possibility of its application to clinical situations as well as basic researches. Further, development of optical imaging may greatly contribute to the understanding of the function of the nervous system.
MeSH Terms
Figure
Reference
-
1. Baker BJ, Kosmidis EK, Vucinic D, Falk CX, Cohen LB, Djurisic M, Zecevic D. Imaging brain activity with voltage-and calcium-sensitive dyes. Cell Mol Neurobiol. 2005. 25:245–282.
Article2. Pouratian N, Cannestra AF, Martin NA, Toga AW. Intraoperative optical intrinsic signal imaging: a clinical tool for functional brain mapping. Neurosurg Focus. 2002. 13:1–9.
Article3. Prakash N, Uhlemann F, Sheth SA, Bookheimer S, Martin N, Toga AW. Current trends in intraoperative optical imaging for functional brain mapping and delineation of lesions of language cortex. Neuroimage. 2008. (in press).
Article4. Aitken PG, Fayuk D, Somjen GG, Turner DA. Use of intrinsic optical signals to monitor physiological changes in brain tissue slices. Methods. 1999. 18:91–103.
Article5. Roe AW. Long-term optical imaging of intrinsic signals in anesthetized and awake monkeys. Appl Opt. 2007. 46:1872–1880.
Article6. Grinvald A, Hildesheim R. VSDI: a new era in functional imaging of cortical dynamics. Nat Rev Neurosci. 2004. 5:874–885.
Article7. Mrsic-Flogel T, Hübener M, Bonhoeffer T. Brain mapping: new wave optical dispatch imaging. Curr Biol. 2003. 13:R778–R780.8. Hill DK, Keynes RD. Opacity changes in stimulated nerve. J Physiol. 1949. 108:278–281.
Article9. Narayan SM, Santori EM, Blood AJ, Burton JS, Toga AW. Imaging optical reflectance in rodent barrel and forelimb sensory cortex. Neuroimage. 1994. 1:181–190.
Article10. Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991. 353:429–431.
Article11. Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA. 1990. 87:6082–6086.
Article12. Grinvald A, Frostig RD, Siegel RM, Bartfeld E. High-resolution optical imaging of functional brain architecture in the awake monkey. Proc Natl Acad Sci USA. 1991. 88:11559–11563.
Article13. Haglund MM, Ojemann GA, Blasdel GG. Optical imaging of bipolar cortical stimulation. J Neurosurg. 1993. 78:785–793.
Article14. Sasaki S, Yazawa I, Miyakawa N, Mochida H, Shinomiya K, Kamino K, Momose-Sato Y, Sato K. Optical imaging of intrinsic signals induced by peripheral nerve stimulation in the in vivo rat spinal cord. Neuroimage. 2002. 17:1240–1255.
Article15. Haglund MM, Ojemann GA, Hochman DW. Optical imaging of epileptiform and functional activity in human cerebral cortex. Nature. 1992. 358:668–671.
Article16. Cannestra AF, Pouratian N, Shomer MH, Toga AW. Refractory periods observed by intrinsic signal and fluorescent dye imaging. J Neurophysiol. 1998. 80:1522–1532.
Article17. Cannestra AF, Bookheimer SY, Pouratian N, O'Farrell A, Sicotte N, Martin NA, Becker D, Rubino G, Toga AW. Temporal and topographical characterization of language cortices using intraoperative optical intrinsinc signals. Neuroimage. 2000. 12:41–54.
Article18. Pouratian N, Bookheimer SY, O'Farrell AM, Sicotte NL, Cannestra AF, Becker D, Toga AW. Optical imaging of bilingual cortical representations. Case report. J Neurosurg. 2000. 93:676–681.19. Cohen LB, Salzberg BM, Davila HV, Ross WN, Landowne D, Waggoner AS, Wang CH. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974. 19:1–36.
Article20. Waggoner AS, Grinvald A. Mechanisms of rapid optical changes of potential sensitive dyes. Ann N Y Acad Sci. 1977. 303:217–242.21. Waggoner AS. Dye indicators of membrane potential. Annu Rev Biophys Bioeng. 1979. 8:47–63.
Article22. Tasaki I, Watanabe A, Sandlin R, Carnay L. Changes in fluorescence, turbidity and birefringence associated with nerve excitation. Proc Natl Acad Sci USA. 1968. 61:883–888.
Article23. Salzberg BM, Davila HV, Cohen LB. Optical recording of impulses in individual neurons of an invertebrate central nervous system. Nature. 1973. 246:508–509.
Article24. Salzberg BM, Grinvald A, Cohen LB, Davila HV, Ross WN. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous recording from several neurons. J Neurophysiol. 1977. 40:1281–1291.
Article25. Tasaki I, Warashina A. Dye-membrane interaction and its changes during nerve excitation. Photochem Photobiol. 1976. 24:191–207.
Article26. Grinvald A, Cohen LB, Lesher S, Boyle MB. Simultaneous optical monitoring of activity of many neurons in invertebrate ganglia, using a 124 element photodiode array. J Neurophysiol. 1981a. 45:829–840.
Article27. Grinvald A, Ross WN, Farber I. Simultaneous optical measurements of electrical activity from multiple sites on processes of cultured neurons. Proc Natl Acad Sci USA. 1981b. 78:3245–3249.
Article28. Grinvald A, Manker A, Segal M. Visualization of the spread of electrical activity in rat hippocampal slices by voltage sensitive optical probes. J Physiol. 1982. 333:269–291.
Article29. Orbach HS, Cohen LB. Simultaneous optical monitoring of activity from many areas of the salamander olfactory bulb. A new method for studying functional organization in the vertebrate CNS. J Neurosci. 1983. 3:2251–2262.
Article30. Petersen CCH, Grinvald A, Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci. 2003. 23:1298–1309.
Article31. Petersen CH, Grinvald A, Sakmann B. Spatio-temporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and anatomical reconstructions. J Neurosci. 2003. 23:1298–1309.
Article32. Kaltenbach JA, Zhang JS. In vivo optical imaging of tone-evoked activity in the dorsal cochlear nucleus with a voltage sensitive dye. J Neurosci Res. 2004. 78:908–917.
Article33. Hosokawa Y, Sugimoto S, Kubota M, Taniguchi I, Horikawa J. Optical imaging of binaural interaction in multiple fields of the guinea pig auditory cortex. Neuroreport. 2004. 15:1093–1097.
Article34. Onimaru H, Homma I. A novel functional neuron group for respiratory thythm generation in the ventral medulla. J Neurosci. 2003. 23:1478–1486.
Article35. Onimaru H, Homma I. Optical imaging of respiratory neuron activity from the dorsal view of the lower brainstem. Clin Exp Pharmacol Physiol. 2005. 32:297–301.
Article36. Sato K, Nariai T, Tanaka Y, Maehara T, Miyakawa N, Sasaki S, Momose-Sato Y, Ohno K. Functional representation of the finger and face in the human somatosensory cortex: intraoperative intrinsic optical imaging. Neuroimage. 2005. 25:1292–1301.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status of Optical Imaging for Evaluating Lymph Nodes and Lymphatic System
- Magnetic Resonance Imaging Meets Fiber Optics: a Brief Investigation of Multimodal Studies on Fiber OpticsBased Diagnostic / Therapeutic Techniques and Magnetic Resonance Imaging
- Photoacoustic imaging platforms for multimodal imaging
- Intraoperative Monitoring and Mapping of the Functional Integrity of the Brainstem
- PET-Based Molecular Nuclear Neuro-Imaging