Endocrinol Metab.
2012 Mar;27(1):72-76. 10.3803/EnM.2012.27.1.72.
A Case of Bilateral Struma Ovarii Combined with Subclinical Hyperthyroidism
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea. injkim@pusan.ac.kr
- KMID: 2134822
- DOI: http://doi.org/10.3803/EnM.2012.27.1.72
Abstract
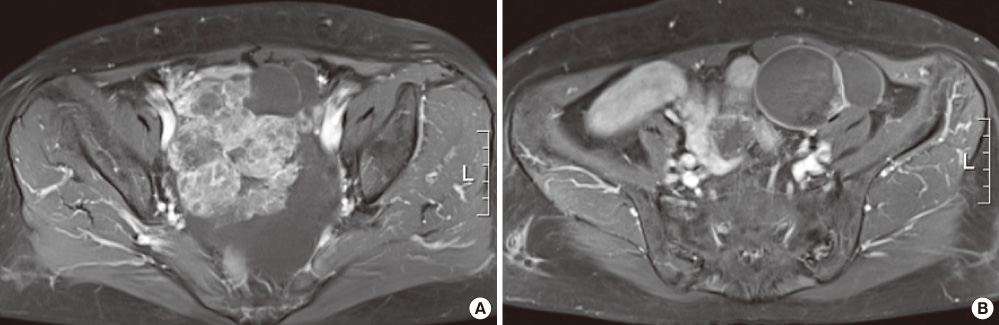
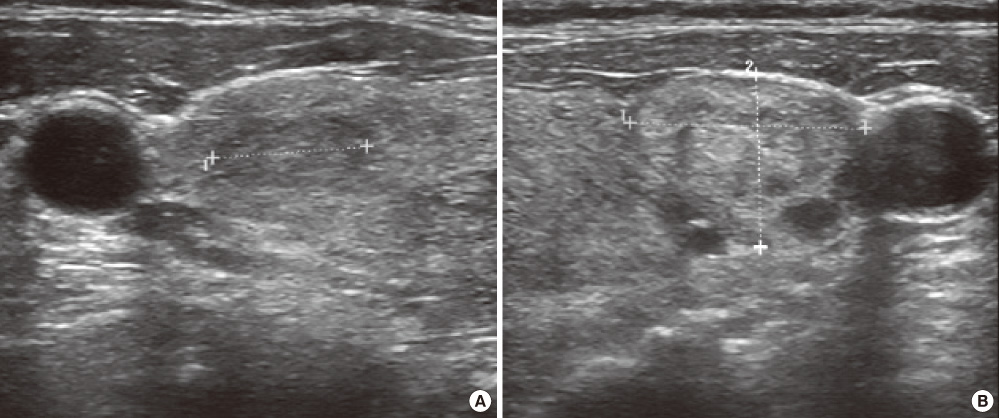
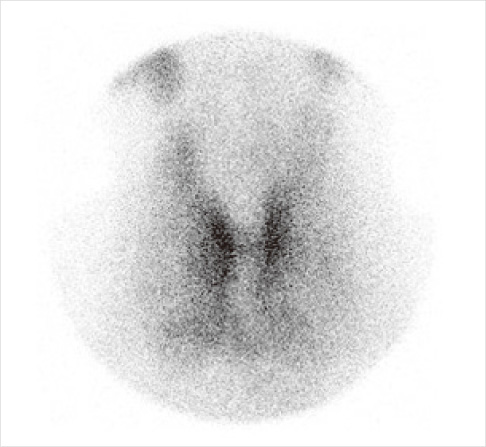
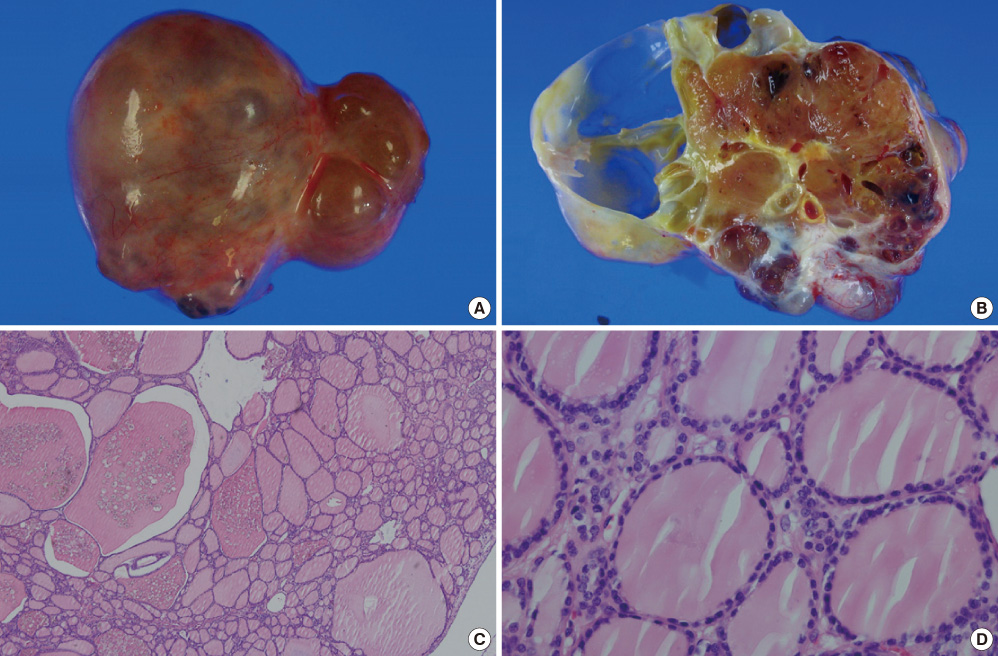
- Struma ovarii is a rare monodermal variant of ovarian teratoma accounting for only 2% of all mature teratomas. To be classified as a struma ovarii, teratoma must be composed predominantly of mature thyroid tissue (> 50%). This tumor is generally benign, although malignant transformation has been reported. Struma ovarii occur mostly as unilateral cases, so bilateral cases are quite rare (less than 6% of cases). Struma ovarii occur largely without symptoms or are accompanied by non-specific symptoms, such as abdominal pain, a palpable abdominal mass, and abdominal distension. The preoperative diagnosis is generally difficult. The incidence of hyperthyroidism has been reported to be 5-10% of patients with struma ovarii. Thus, cases of functional bilateral struma ovarii are very rare. We report a case of bilateral struma ovarii with subclinical thyrotoxicosis and a diffuse goiter, mimicking a malignant ovarian tumor, and include a brief review of related literature.
MeSH Terms
Figure
Reference
-
1. Dardik RB, Dardik M, Westra W, Montz FJ. Malignant struma ovarii: two case reports and a review of the literature. Gynecol Oncol. 1999. 73:447–451.2. Gould SF, Lopez RL, Speers WC. Malignant struma ovarii. A case report and literature review. J Reprod Med. 1983. 28:415–419.3. Fleuren GJ, Coerkamp EG, Nap M, vd Broek LJ, Warnaar SO. Immunohistological characterization of a monoclonal antibody (OV632) against epithelial ovarian carcinomas. Virchows Arch A Pathol Anat Histopathol. 1987. 410:481–486.4. Woodruff JD, Rauh JT, Markley RL. Ovarian struma. Obstet Gynecol. 1966. 27:194–201.5. Hasleton PS, Kelehan P, Whittaker JS, Burslem RW, Turner L. Benign and malignant struma ovarii. Arch Pathol Lab Med. 1978. 102:180–184.6. Pardo-Mindan FJ, Vazquez JJ. Malignant struma ovarii. Light and electron microscopic study. Cancer. 1983. 51:337–343.7. Ayhan A, Yanik F, Tuncer R, Tuncer ZS, Ruacan S. Struma ovarii. Int J Gynaecol Obstet. 1993. 42:143–146.8. Smith FC. Pathology and physiology of struma ovarii. Arch Surg. 1946. 53:603–626.9. Grandet PJ, Remi MH. Struma ovarii with hyperthyroidism. Clin Nucl Med. 2000. 25:763–765.10. Kim SJ, Pak K, Lim HJ, Yun KH, Seong SJ, Kim TJ, Lim KT, Jung HW, Park IS, Shim JU, Park CT, Lee KH. Clinical diversity of struma ovarii. Korean J Obstet Gynecol. 2002. 45:748–752.11. Kempers RD, Dockerty MB, Hoffman DL, Bartholomew LG. Struma ovarii: ascitic, hyperthyroid, and asymptomatic syndromes. Ann Intern Med. 1970. 72:883–893.12. Devaney K, Snyder R, Norris HJ, Tavassoli FA. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynecol Pathol. 1993. 12:333–343.13. Rosenblum NG, LiVolsi VA, Edmonds PR, Mikuta JJ. Malignant struma ovarii. Gynecol Oncol. 1989. 32:224–227.14. O'Connell ME, Fisher C, Harmer CL. Malignant struma ovarii: presentation and management. Br J Radiol. 1990. 63:360–363.15. Berghella V, Ngadiman S, Rosenberg H, Hoda S, Zuna RE. Malignant struma ovarii. A case report and review of the literature. Gynecol Obstet Invest. 1997. 43:68–72.16. Ronga G, Fiorentino A, Paserio E, Signore A, Todino V, Tummarello MA, Filesi M, Baschieri I. Can iodine-131 whole-body scan be replaced by thyroglobulin measurement in the post-surgical follow-up of differentiated thyroid carcinoma? J Nucl Med. 1990. 31:1766–1771.17. Lubin E, Mechlis-Frish S, Zatz S, Shimoni A, Segal K, Avraham A, Levy R, Feinmesser R. Serum thyroglobulin and iodine-131 whole-body scan in the diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. J Nucl Med. 1994. 35:257–262.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Malignant struma ovarii with hyperthyroidism ; radionuclide study and treatment
- Struma Ovarii: A Case of Struma Ovarii and Literature Review
- Struma Ovarii Showing the Clinical Characteristics of the Malignancy
- A Case of Struma Ovarii
- Malignant Struma Ovarii With Graves’ Disease and Papillary Thyroid Carcinoma: A Case Report