Endocrinol Metab.
2012 Mar;27(1):12-19. 10.3803/EnM.2012.27.1.12.
Mechanism of Lipid Induced Insulin Resistance: An Overview
- Affiliations
-
- 1Molecular Endocrinology Laboratory, Centre for Advanced Studies in Zoology, Visva-Bharati University School of Life Science, Santiniketan, India. bhattacharyasa@gmail.com
- 2North-East Institute of Science & Technology (CSIR), Jorhat, India.
- KMID: 2134812
- DOI: http://doi.org/10.3803/EnM.2012.27.1.12
Abstract
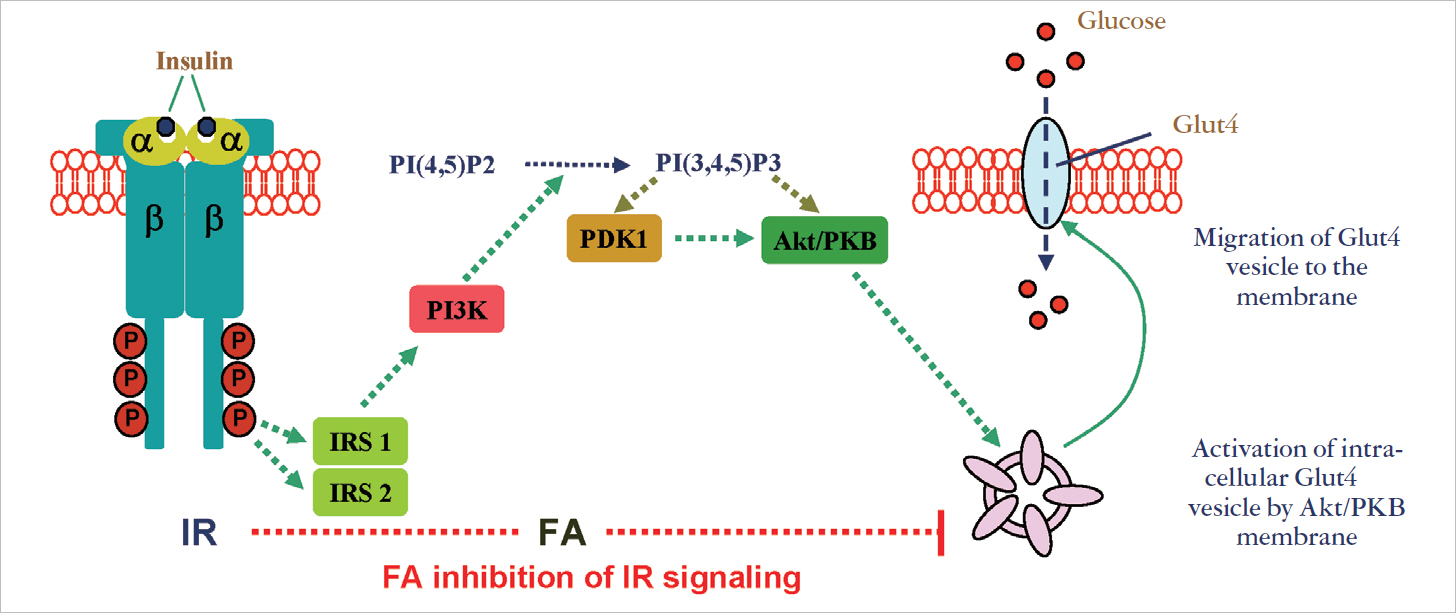
- Type 2 diabetes (T2D) is rapidly spreading throughout the world. It's an insidious disease and still treated in an indirect manner without having specific drug target. In majority cases T2D is treated with drugs that address type 1 diabetes, majority of drugs aim to increase insulin release although the root cause for T2D is not the dearth of insulin release, it occurs in the later stage of disease development. T2D silently progressed in the patient; it begins with insulin resistance that takes place due to the loss of insulin sensitivity. Though insulin resistance is the centre of pathogenesis, our treatment of the disease has not yet addressed it. It is now a fact that insulin resistance is manifested by lipid and fatty acids (FAs) play a critical role in blunting insulin sensitivity. Our understanding is still poor in deciphering how lipid impose insulin insensitivity, majority of workers suggest it is because of insulin signaling defects which implements insulin function in inhibiting glucose to the cell from circulation. Number of long chain saturated FA has been shown to produce insulin signaling defects. However, we really need further investigation to find specific target(s) for FA induced damage. In addition to these information, a new dimension of T2D is getting attractive is fetuin-A/alpha2-Heremans-Schmid Glycoprotein, a secretary protein from liver. Its gene locus has been identified as T2D susceptible. Fetuin-A's excess expression occurs by FA and it disrupts adipocyte function. It has been shown to be associated with T2D especially in obesity. In this review, we briefly discuss the present status on the mechanistic understanding of lipid induced insulin resistance that leads to T2D. More we understand the mechanism; opportunity to fight the battle with T2D will be increasing. Since, this field is now vast; we covered a few major events.
Keyword
MeSH Terms
Figure
Reference
-
1. Kolterman OG, Reaven GM, Olefsky JM. Relationship between in vivo insulin resistance and decreased insulin receptors in obese man. J Clin Endocrinol Metab. 48:487–494. 1979.
Article2. Le Marchand-Brustel Y, Grémeaux T, Ballotti R, Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resis-tant obese mice. Nature. 315:676–679. 1985.
Article3. Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 46:3–10. 1997.
Article4. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 51:7–18. 2002.5. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: de-fining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32 Suppl. 3:14–23. 2002.6. Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 48:1836–1841. 1999.
Article7. Zimmet PZ. Diabetes epidemiology as a tool to trigger diabetes research and care. Diabetologia. 42:499–518. 1999.
Article8. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 414:782–787. 2001.
Article9. Ruddock MW, Stein A, Landaker E, Park J, Cooksey RC, McClain D, Patti ME. Saturated fatty acids inhibit hepatic insulin action by modulat-ing insulin receptor expression and post-receptor signalling. J Biochem. 144:599–607. 2008.
Article10. Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desatura-tion and elongation of Fatty acids and insulin action. Ann N Y Acad Sci. 967:183–195. 2002.
Article12. Hennes MM, Shrago E, Kissebah AH. Receptor and postreceptor effects of free fatty acids (FFA) on hepatocyte insulin dynamics. Int J Obes. 14:831–841. 1990.11. Haag M, Dippenaar NG. Dietary fats, fatty acids and insulin resistance: short review of a multifaceted connection. Med Sci Monit. 11:RA359–RA367. 2005.13. Wyne KL. Free fatty acids and type 2 diabetes mellitus. Am J Med. 115(Suppl 8A):29S–36S. 2003.
Article14. Rosen OM. After insulin binds. Science. 237:1452–1458. 1987.
Article15. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 414:799–806. 2001.
Article16. Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular tar-gets of insulin resistance. J Clin Invest. 106:165–169. 2000.
Article17. Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 8:55–62. 1998.
Article18. Kupriyanova TA, Kandror KV. Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. J Biol Chem. 274:1458–1464. 1999.
Article19. Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and altera-tions in the insulin signaling cascade. Diabetes. 48:1270–1274. 1999.
Article20. Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 277:50230–50236. 2002.
Article21. Dey D, Mukherjee M, Basu D, Datta M, Roy SS, Bandyopadhyay A, Bhat-tacharya S. Inhibition of insulin receptor gene expression and insulin signaling by fatty acid: interplay of PKC isoforms therein. Cell Physiol Biochem. 16:217–228. 2005.
Article22. Dey D, Basu D, Roy SS, Bandyopadhyay A, Bhattacharya S. Involvement of novel PKC isoforms in FFA induced defects in insulin signaling. Mol Cell Endocrinol. 246:60–64. 2006.
Article23. Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposi-tion and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 85:662–677. 2007.24. Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 93:2438–2446. 1994.
Article25. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 106:171–176. 2000.
Article26. Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 103:253–259. 1999.
Article27. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jen-kins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 46:983–988. 1997.
Article28. Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 51:2005–2011. 2002.29. Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 52:1926–1934. 2003.30. Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Sum-mers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 278:10297–10303. 2003.
Article31. Reynoso R, Salgado LM, Calderón V. High levels of palmitic acid lead to insulin resistance due to changes in the level of phosphorylation of the insulin receptor and insulin receptor substrate-1. Mol Cell Biochem. 246:155–162. 2003.
Article32. Martin S, Millar CA, Lyttle CT, Meerloo T, Marsh BJ, Gould GW, James DE. Effects of insulin on intracellular GLUT4 vesicles in adipocytes: evidence for a secretory mode of regulation. J Cell Sci 113 Pt. 19:3427–3438. 2000.
Article33. Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 274:2593–2596. 1999.
Article34. Dey D, Bhattacharya A, Roy S, Bhattacharya S. Fatty acid represses insulin receptor gene expression by impairing HMGA1 through protein kinase Cepsilon. Biochem Biophys Res Commun. 357:474–479. 2007.35. Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 281:2042–2045. 1998.
Article36. Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 370(Pt 2):361–371. 2003.
Article37. Strack V, Stoyanov B, Bossenmaier B, Mosthaf L, Kellerer M, Häring HU. Impact of mutations at different serine residues on the tyrosine kinase activity of the insulin receptor. Biochem Biophys Res Commun. 239:235–239. 1997.
Article38. Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Insulin induces specific interaction between insulin receptor and protein kinase C delta in primary cultured skeletal muscle. Mol Endocrinol. 15:565–574. 2001.39. Greene MW, Morrice N, Garofalo RS, Roth RA. Modulation of human insulin receptor substrate-1 tyrosine phosphorylation by protein kinase Cdelta. Biochem J. 378(Pt 1):105–116. 2004.
Article40. Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 274:24202–24210. 1999.
Article41. Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, Manfioletti G, Barbetti F, Brunetti A, Croce CM, Fusco A. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat Med. 11:765–773. 2005.
Article42. Lastres-Becker I, Brodesser S, Lütjohann D, Azizov M, Buchmann J, Hin-termann E, Sandhoff K, Schürmann A, Nowock J, Auburger G. Insulin receptor and lipid metabolism pathology in ataxin-2 knock-out mice. Hum Mol Genet. 17:1465–1481. 2008.
Article43. Ikeda Y, Olsen GS, Ziv E, Hansen LL, Busch AK, Hansen BF, Shafrir E, Mosthaf-Seedorf L. Cellular mechanism of nutritionally induced insulin resistance in Psammomys obesus: overexpression of protein kinase Cepsilon in skeletal muscle precedes the onset of hyperinsulinemia and hyper-glycemia. Diabetes. 50:584–592. 2001.44. Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 117:739–745. 2007.45. Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 6:87–97. 2000.
Article46. Mack E, Ziv E, Reuveni H, Kalman R, Niv MY, Jörns A, Lenzen S, Shafrir E. Prevention of insulin resistance and beta-cell loss by abrogating PK-Cepsilon-induced serine phosphorylation of muscle IRS-1 in Psammomys obesus. Diabetes Metab Res Rev. 24:577–584. 2008.47. Dasgupta S, Bhattacharya S, Maitra S, Pal D, Majumdar SS, Datta A. Mechanism of lipid induced insulin resistance: activated PKCε is a key regulator. Biochim Biophys Acta. 1812:495–506. 2011.
Article48. Foti D, Iuliano R, Chiefari E, Brunetti A. A nucleoprotein complex con-taining Sp1, C/EBP beta, and HMGI-Y controls human insulin receptor gene transcription. Mol Cell Biol. 23:2720–2732. 2003.49. Harrer M, Lührs H, Bustin M, Scheer U, Hock R. Dynamic interaction of HMGA1a proteins with chromatin. J Cell Sci. 117(Pt 16):3459–3471. 2004.
Article50. Brunetti A, Manfioletti G, Chiefari E, Goldfine ID, Foti D. Transcription-al regulation of human insulin receptor gene by the high-mobility group protein HMGI(Y). FASEB J. 15:492–500. 2001.
Article51. Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Leprêtre F, Lecoeur C, Gallina P, Zekiri L, Dina C, Froguel P. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet. 67:1470–1480. 2000.
Article52. Auberger P, Falquerho L, Contreres JO, Pages G, Le Cam G, Rossi B, Le Cam A. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 58:631–640. 1989.
Article53. Rauth G, Pöschke O, Fink E, Eulitz M, Tippmer S, Kellerer M, Häring HU, Nawratil P, Haasemann M, Jahnen-Dechent W, Müller-Esterl W. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem. 204:523–529. 1992.
Article54. Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, Jen KL, Charron MJ, Jahnen-Dechent W, Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 51:2450–2458. 2002.
Article55. Mori K, Emoto M, Yokoyama H, Araki T, Teramura M, Koyama H, Shoji T, Inaba M, Nishizawa Y. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care. 29:468. 2006.
Article56. Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Häring HU, Schulze MB. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 57:2762–2767. 2008.
Article57. Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG. Health ABC Study. Fetuin-A and incident diabetes mellitus in older persons. JAMA. 300:182–188. 2008.
Article58. Chen NX, O'Neill KD, Chen X, Duan D, Wang E, Sturek MS, Edwards JM, Moe SM. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am J Physiol Renal Physiol. 292:F599–F606. 2007.
Article59. Lin X, Braymer HD, Bray GA, York DA. Differential expression of insulin receptor tyrosine kinase inhibitor (fetuin) gene in a model of diet-induced obesity. Life Sci. 63:145–153. 1998.
Article60. Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Kröber SM, Machicao F, Fritsche A, Häring HU. Alpha2-Heremans-Schmid glycoprotein/ fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 29:853–857. 2006.61. Dahlman I, Eriksson P, Kaaman M, Jiao H, Lindgren CM, Kere J, Arner P. alpha2-Heremans-Schmid glycoprotein gene polymorphisms are associated with adipocyte insulin action. Diabetologia. 47:1974–1979. 2004.62. Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Häring HU, Stefan N. Fetuin-A induces cytokine expression and suppresses adiponec-tin production. PLoS One. 3:e1765. 2008.
Article63. Dasgupta S, Bhattacharya S, Biswas A, Majumdar SS, Mukhopadhyay S, Ray S. NF-kappaB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochem J. 429:451–462. 2010.64. Barma P, Bhattacharya S, Bhattacharya A, Kundu R, Dasgupta S, Biswas A, Roy SS. Lipid induced overexpression of NF-kappaB in skeletal muscle cells is linked to insulin resistance. Biochim Biophys Acta. 1792:190–200. 2009.65. Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a trans-membrane protein. Cell. 52:269–279. 1988.
Article66. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dor-soventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 86:973–983. 1996.
Article67. Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 16:3–9. 2004.
Article68. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 112:1796–1808. 2003.
Article69. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 112:1821–1830. 2003.
Article70. Chait A, Kim F. Saturated fatty acids and inflammation: who pays the toll? Arterioscler Thromb Vasc Biol. 30:692–693. 2010.
Article71. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links in-nate immunity and fatty acid-induced insulin resistance. J Clin Invest. 116:3015–3025. 2006.
Article72. Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, Tanti JF, Burcelin R, Alessi MC. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 50:1267–1276. 2007.
Article73. Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 56:1986–1998. 2007.
Article74. Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 346:739–745. 2006.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin Resistance: From Mechanisms to Therapeutic Strategies
- Inflammation and Insulin Resistance: An Old Story with New Ideas
- The mechanism of development of insulin resistance in type 2 diabetes mellitus
- Potential Therapeutic Strategies for Fat Induced Insulin Resistance
- Insulin Resistance and Insulin Resistance Syndrome