Clin Exp Otorhinolaryngol.
2012 Sep;5(3):122-131. 10.3342/ceo.2012.5.3.122.
Binaural Electric-Acoustic Interactions Recorded from the Inferior Colliculus of Guinea Pigs: The Effect of Masking Observed in the Central Nucleus of the Inferior Colliculus
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, St. Vincent's Hospital, The Catholic University of Korea School of Medicine, Suwon, Korea.
- 2Department of Otolaryngology-Head and Neck Surgery, Uijeongbu St. Mary's Hospital, The Catholic University of Korea School of Medicine, Uijeongbu, Korea. leedh0814@catholic.ac.kr
- KMID: 2134594
- DOI: http://doi.org/10.3342/ceo.2012.5.3.122
Abstract
OBJECTIVES
To investigate the electric-acoustic interactions within the inferior colliculus of guinea pigs and to observe how central masking appears in invasive neural recordings of the inferior colliculus (IC).
METHODS
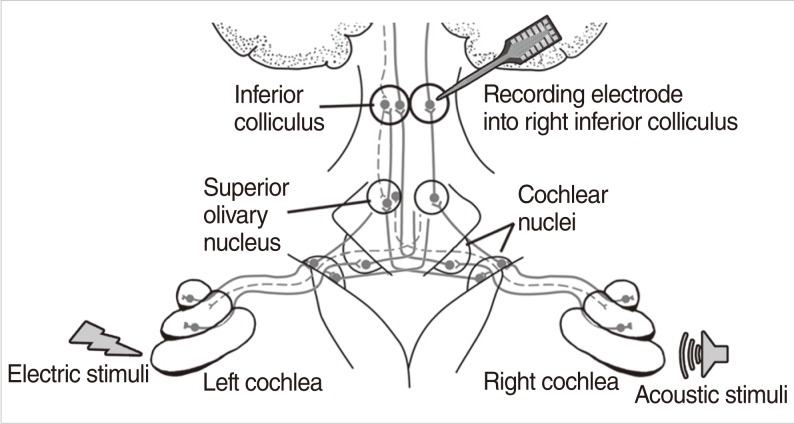
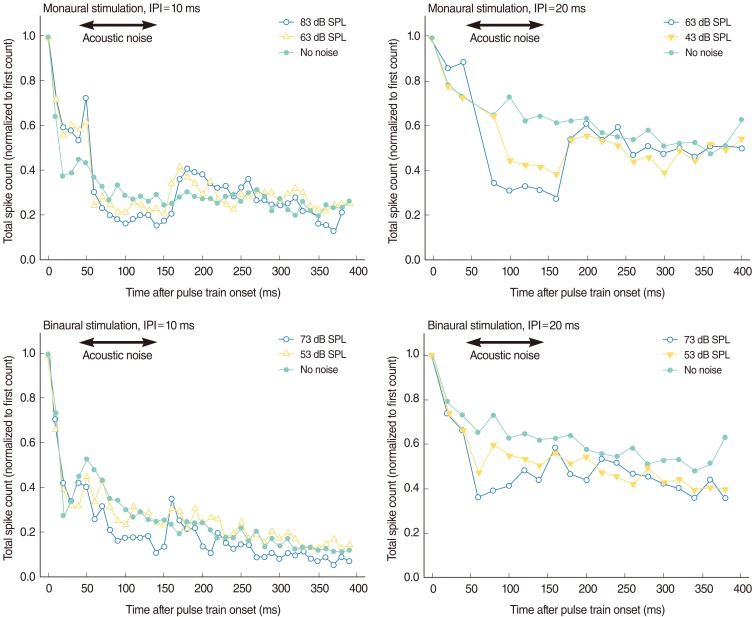
A platinum-iridium wire was inserted to scala tympani through cochleostomy with a depth no greater than 1 mm for intracochlear stimulation of electric pulse train. A 5 mm 100 microm, single-shank, thin-film, penetrating recording probe was inserted perpendicularly to the surface of the IC in the coronal plane at an angle of 30-40degrees off the parasagittal plane with a depth of 2.0-2.5 mm. The peripheral and central masking effects were compared using electric pulse trains to the left ear and acoustic noise to the left ear (ipsilateral) and to the right ear (contralateral). Binaural acoustic stimuli were presented with different time delays and compared with combined electric and acoustic stimuli. The averaged evoked potentials and total spike numbers were measured using thin-film electrodes inserted into the central nucleus of the IC.
RESULTS
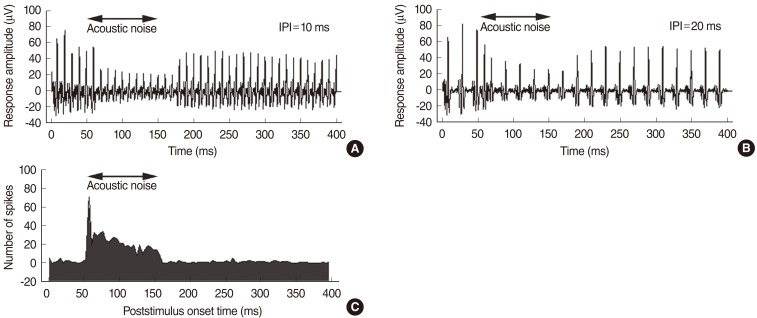
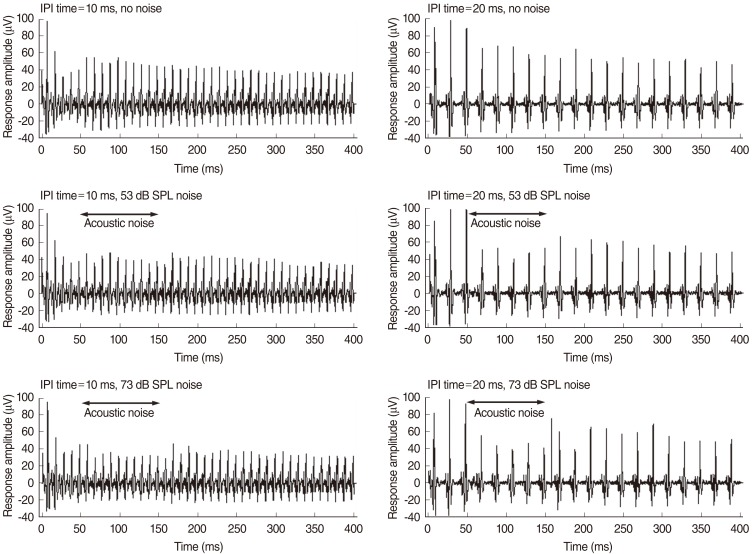
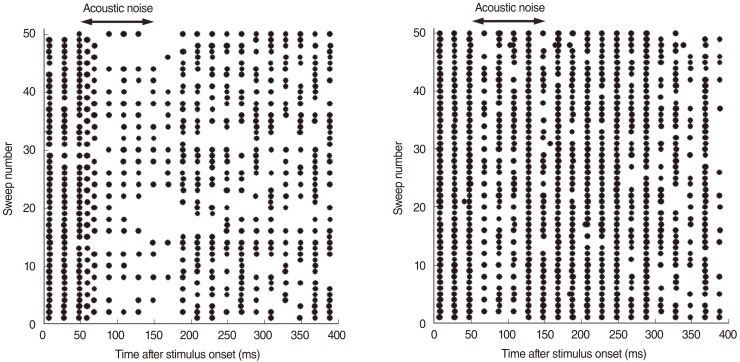
Ipsilateral noise had more obvious effects on the electric response than did contralateral noise. Contralateral noise decreased slightly the response amplitude to the electric pulse train stimuli. Immediately after the onset of acoustic noise, the response pattern changed transiently with shorter response intervals. The effects of contralateral noise were evident at the beginning of the continuous noise. The total spike number decreased when the binaural stimuli reached the IC most simultaneously.
CONCLUSION
These results suggest that central masking is quite different from peripheral masking and occurs within the binaural auditory system, and this study showed that the effect of masking could be observed in the IC recording. These effects are more evident and consistent with the psychophysical data from spike number analyses than with the previously reported gross potential data.
Keyword
MeSH Terms
Figure
Reference
-
1. Seeber BU. Havelock D, Kuwano S, Vorlander M, editors. Masking and critical bands. Handbook of signal processing in acoustics. 2008. New York: Springer;p. 229–240.
Article2. Zwislocki JJ. A theory of central auditory masking and its partial validation. J Acoust Soc Am. 1972; 52(2B):644–659.
Article3. Zwislocki JJ. Central masking and neural activity in the cochlear nucleus. Audiology. 1971; Jan-Feb. 10(1):48–59. PMID: 5163654.
Article4. Aran JM, Pajor AM, de Sauvage RC, Erre JP. Role of the efferent medial olivocochlear system in contralateral masking and binaural interactions: an electrophysiological study in guinea pigs. Audiology. 2000; Nov-Dec. 39(6):311–321. PMID: 11766691.5. Zhang F, Boettcher FA, Sun XM. Contralateral suppression of distortion product otoacoustic emissions: effect of the primary frequency in Dpgrams. Int J Audiol. 2007; 4. 46(4):187–195. PMID: 17454232.
Article6. Palmer AR, Jiang D, McAlpine D. Desynchronizing responses to correlated noise: a mechanism for binaural masking level differences at the inferior colliculus. J Neurophysiol. 1999; 2. 81(2):722–734. PMID: 10036273.
Article7. Champoux F, Paiement P, Mercier C, Lepore F, Lassonde M, Gagne JP. Auditory processing in a patient with a unilateral lesion of the inferior colliculus. Eur J Neurosci. 2007; 1. 25(1):291–297. PMID: 17241290.
Article8. Cranford JL. Role of neocortex in binsural hearing in the cat: I. contralateral masking. Brain Res. 1975; 12. 100(2):395–406. PMID: 1192183.9. Harris J. Brain lesions, central masking, and dichotic speech perception. Brain Lang. 1994; 1. 46(1):96–108. PMID: 8131046.
Article10. Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol. 2004; 9. 5(3):305–322. PMID: 15492888.
Article11. Abbas PJ, Noh H, Jeng FC, Miller CA, Robinson BK, Nourski KV. Effects of remaining hair cells on cochlear implant function. 2004. Iowa: University of Iowa;Contract no.: N01-DC-2-1005.12. Rapisarda C, Bacchelli B. The brain of the guinea pig in stereotaxic coordinates. Arch Sci Biol (Bologna). 1977; Jan-Dec. 61(1-4):1–37. PMID: 400095.13. Snyder RL, Rebscher SJ, Cao KL, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat: I. expansion of central representation. Hear Res. 1990; 12. 50(1-2):7–33. PMID: 2076984.
Article14. Teas DC, Nielsen DW. Interaural attenuation versus frequency for guinea pig and chinchilla CM response. J Acoust Soc Am. 1975; 11. 58(5):1066–1072. PMID: 1194558.
Article15. Marsh RR, Yamane H, Potsic WP. Effect of site of stimulation on the guinea pig's electrically evoked brain stem response. Otolaryngol Head Neck Surg. 1981; Jan-Feb. 89(1):125–130. PMID: 6784070.
Article16. Hu N, Abbas PJ, Miller CA, Robinson BK, Nourski KV, Jeng FC, et al. Auditory response to intracochlear electric stimuli following furosemide treatment. Hear Res. 2003; 11. 185(1-2):77–89. PMID: 14599695.
Article17. Matsuoka AJ, Abbas PJ, Rubinstein JT, Miller CA. The neuronal response to electrical constant-amplitude pulse train stimulation: additive Gaussian noise. Hear Res. 2000; 11. 149(1-2):129–137. PMID: 11033252.
Article18. Snyder R, Leake P, Rebscher S, Beitel R. Temporal resolution of neurons in cat inferior colliculus to intracochlear electrical stimulation: effects of neonatal deafening and chronic stimulation. J Neurophysiol. 1995; 2. 73(2):449–467. PMID: 7760111.
Article19. Oliver DL. Projections to the inferior colliculus from the anteroventral cochlear nucleus in the cat: possible substrates for binaural interaction. J Comp Neurol. 1987; 10. 264(1):24–46. PMID: 2445792.
Article20. Dum N, von Wedel H. Effect of ipsilateral, contralateral and binaural roaring noise on brain stem potentials in the guinea pig. Laryngol Rhinol Otol (Stuttg). 1984; 12. 63(12):636–639. PMID: 6521594.21. Prasher DK, Cohen M. The selective effects of central masking on brain stem potentials. Br J Audiol. 1984; 5. 18(2):79–83. PMID: 6733320.
Article22. Rosenhamer H, Holmkvist C. Will contralateral white noise interfere with the monaurally click-evoked brainstem response? Scand Audiol. 1983; 12(1):11–14. PMID: 6844866.
Article23. Melcher JR, Knudson IM, Fullerton BC, Guinan JJ Jr, Norris BE, Kiang NY. Generators of the brainstem auditory evoked potential in cat: I. an experimental approach to their identification. Hear Res. 1996; 4. 93(1-2):1–27. PMID: 8735066.
Article24. Owen GA, Burkard R. Ipsilateral, contralateral, and binaural masking effects on the human brain-stem auditory-evoked responses to click stimuli. J Acoust Soc Am. 1991; 4. 89(4 Pt 1):1760–1767. PMID: 2045584.
Article25. Dobie RA, Wilson MJ. Binaural interaction in auditory brain-stem responses: effects of masking. Electroencephalogr Clin Neurophysiol. 1985; 1. 62(1):56–64. PMID: 2578378.
Article26. Noh H, Abbas PJ, Abbas CA, Nourski KV, Robinson BK, Jeng FC. Binaural interactions of electrically and acoustically evoked responses recorded from the inferior colliculus of guinea pigs. Int J Audiol. 2007; 6. 46(6):309–320. PMID: 17530515.
Article27. Furst M, Levine RA, McGaffigan PM. Click lateralization is related to the beta component of the dichotic brainstem auditory evoked potentials of human subjects. J Acoust Soc Am. 1985; 11. 78(5):1644–1651. PMID: 4067079.28. Guinan JJ Jr. Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006; 12. 27(6):589–607. PMID: 17086072.
Article29. Guinan JJ Jr. Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg. 2010; 10. 18(5):447–453. PMID: 20717032.
Article30. Brown MC. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res. 1989; 6. 40(1-2):93–109. PMID: 2768087.
Article31. Lilaonitkul W, Guinan JJ Jr. Human medial olivocochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol. 2009; 9. 10(3):459–470. PMID: 19263165.
Article32. Lin P, Turner CW, Gantz BJ, Djalilian HR, Zeng FG. Ipsilateral masking between acoustic and electric stimulations. J Acoust Soc Am. 2011; 8. 130(2):858–865. PMID: 21877801.
Article33. Gott PS, Hughes EC. Effect of noise masking on the brain-stem and middle-latency auditory evoked potentials: central and peripheral components. Electroencephalogr Clin Neurophysiol. 1989; Mar-Apr. 74(2):131–138. PMID: 2465888.
Article34. Galambos R, Makeig S. Physiological studies of central masking in man: I. the effects of noise on the 40-Hz steady-state response. J Acoust Soc Am. 1992; 11. 92(5):2683–2690. PMID: 1479130.
Article35. Galambos R, Makeig S. Physiological studies of central masking in man: II. tonepip SSRs and the masking level difference. J Acoust Soc Am. 1992; 11. 92(5):2691–2697. PMID: 1479131.
Article36. Sagharichi M, Fulton RT. Effect of a contralateral masking stimulus on auditory response performance. J Aud Res. 1983; 4. 23(2):77–93. PMID: 6679550.37. Snyder JM. Central masking in normal listeners. Acta Otolaryngol. 1973; 5. 75(5):419–424. PMID: 4730791.
Article38. Benton SL, Sheeley EC. Effects of three contralateral maskers on pure-tone thresholds using manual audiometry. Audiology. 1987; 26(4):227–234. PMID: 3632477.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of beta-Adrenergic Receptor mRNA in the Visual Cortex and Superior Colliculus of Monocular Deprivated Rat
- Immunoreactivity of Calcium-Binding Proteins in the Central Auditory Nervous System of Aged Rats
- Changes of Calretinin-Immunoreactivities in the Rat Superior Collicuclus after Eye Enucleation
- Calculi in the Prostatic Utricle Communicated with an Ejaculatory Duct
- The distribution of calbindin-D28k, parvalbumin, and calretinin immunoreactivity in the inferior colliculus of circling mouse