J Gynecol Oncol.
2008 Jun;19(2):139-144. 10.3802/jgo.2008.19.2.139.
Tenascin-X and leukemia inhibitory factor receptor are down-regulated in leiomyoma compared with normal myometrium
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ewha Womans University, Seoul, Korea. onco@ewha.ac.kr
- 2Medical Research Institute, School of Medicine, Ewha Womans University, Seoul, Korea.
- KMID: 2129915
- DOI: http://doi.org/10.3802/jgo.2008.19.2.139
Abstract
-
OBJECTIVE
Uterine leiomyomas are the most common tumor of the uterus. But the molecular causes of uterine leiomyoma remain unclear. We conducted the current investigation in order to elucidate the molecular mechanisms in the development of uterine leiomyoma.
METHODS
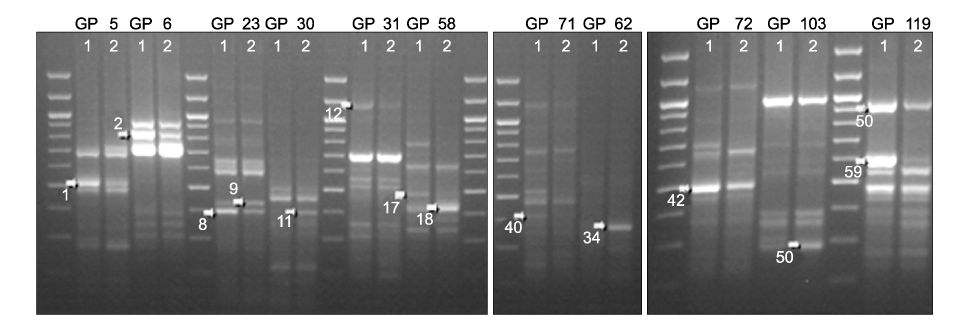
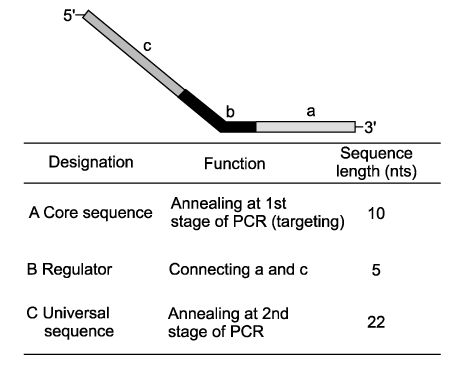
We employed a new and accurate reverse transcription-polymerase chain reaction (RT-PCR) method that involved annealing control primers (ACPs) to identify the genes that are differently expressed in uterine leiomyoma.
RESULTS
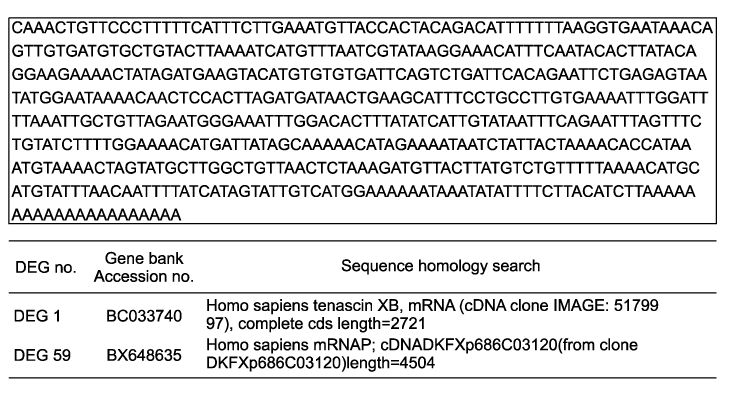
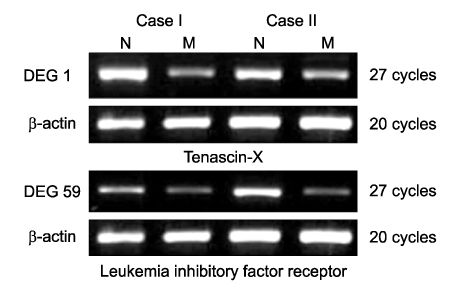
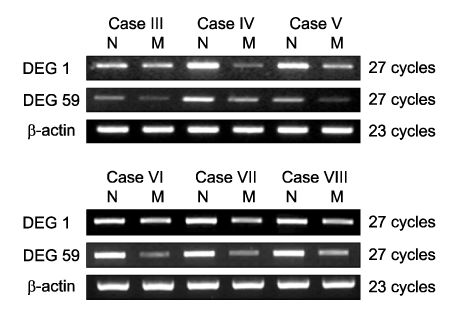
Using 120 ACPs, we identified and sequenced 14 differently expressed genes (DEGs) in uterine leiomyoma compared with normal myometrium. Basic Local Alignment Search Tool (BLAST) searches were performed to examine the known functions of these genes associated with uterine leiomyoma. We confirmed differently expressed patterns in more cases using the RT-PCR method. We also detected two novel genes, Tenascin-X and Leukemia Inhibitory Factor Receptor (LIFR), which had not yet been reported to have any functions associated with uterine leiomyoma. RT-PCR confirmation shows that both of these two genes are down-regulated in uterine leiomyoma.
CONCLUSION
Our results suggest that Tenascin-X and LIFR may play a role in the development of uterine leiomyoma. Although further studies are required to establish the precise mechanisms with which these genes are involved in the genesis of uterine leiomyoma, the present research is significant in that it is the first study which detects down-regulated novel genes in uterine leiomyoma using the ACP system.
Keyword
MeSH Terms
Figure
Reference
-
1. Pokras R, Hufnagel VG. Hysterectomies in the United States 1965-84. 1987. Hyattsville, MD: US Department of Health and Human Services, Public Health Service, CDC;(Vital and health statistics; series 13, no. 92). DHHS publication no. (PHS) 88-1753.2. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: A double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991. 77:720–725.3. Brandon DD, Erickson TE, Keenan EJ, Strawn EY, Novy MJ, Burry KA, et al. Estrogen receptor gene expression in human uterine leiomyomata. J Clin Endocrinol Metab. 1995. 80:1876–1881.4. Yeh J, Rein M, Nowak R. Presence of messenger ribonucleic acid for epidermal growth factor (EGF) and EGF receptor demonstrable in monolayer cell cultures of myometria and leiomyomata. Fertil Steril. 1991. 56:997–1000.5. Pandis N, Heim S, Bardi G, Flodérus UM, Willén H, Mandahl N, et al. Chromosome analysis of 96 uterine leiomyomas. Cancer Genet Cytogenet. 1991. 55:11–18.6. Ishwad CS, Ferrell RE, Davare J, Meloni AM, Sandberg AA, Surti U. Molecular and cytogenetic analysis of chromosome 7 in uterine leiomyomas. Genes Chromosomes Cancer. 1995. 14:51–55.7. Wanschura S, Belge G, Stenman G, Kools P, Dal Cin P, Schoenmakers E, et al. Mapping of the translocation breakpoints of primary pleomorphic adenomas and lipomas within a common region of chromosome 12. Cancer Genet Cytogenet. 1996. 86:39–45.8. Wan JS, Sharp SJ, Poirier GM, Wagaman PC, Chambers J, Pyati J. Cloning differentially expressed mRNAs. Nat Biotechnol. 1996. 14:1685–1691.9. Kim YJ, Kwak CI, Gu YY, Hwang IT, Chun JY. Annealing control primer system for identification of differentially expressed genes on agarose gels. Biotechniques. 2004. 36:424–434.10. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990. 215:403–410.11. Li B, Sun M, He B, Yu J, Zhang YD, Zhang YL. Identification of differentially expressed genes in human uterine leiomyomas using differential display. Cell Res. 2002. 12:39–45.12. Skubitz KM, Skubitz AP. Differential gene expression in uterine leiomyoma. J Lab Clin Med. 2003. 141:297–308.13. Debouck C. Differential display or differential dismay? Curr Opin Biotechnol. 1995. 6:597–599.14. Liang P, Pardee AB. Recent advances in differential display. Curr Opin Immunol. 1995. 7:274–280.15. Hwang KC, Cui XS, Park SP, Shin MR, Park SY, Kim EY, et al. Identification of differentially regulated genes in bovine blastocysts using an annealing control primer system. Mol Reprod Dev. 2004. 69:43–51.16. Chiquet-Ehrismann R. Tenascins. Int J Biochem Cell Biol. 2004. 36:986–990.17. Chiquet-Ehrismann R, Tucker RP. Connective tissues: Signalling by tenascins. Int J Biochem Cell Biol. 2004. 36:1085–1089.18. Gearing DP, Gough NM, King JA, Hilton DJ, Nicola NA, Simpson RJ, et al. Molecular cloning and expression of cDNA encoding a murine myeloid leukemia inhibitory factor (LIF). EMBO. 1987. 6:3995–4002.19. Turnley AM, Bartlett PF. Cytokines that signal through the leukemia inhibitory factor receptor-beta complex in the nervous system. J Neurochem. 2000. 74:889–899.20. McKenzie RC, Szepietowski J. Cutaneous leukemia inhibitory factor and its potential role in the development of skin tumors. Dermatol Surg. 2004. 30:279–290.21. Tanaka M, Miyajima A. Oncostatin M, a multifunctional cytokine. Rev Physiol Biochem Pharmacol. 2003. 149:39–52.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Analysis of Estrogen Receptors and Progesterone Receptors in Leiomyoma of Uterus Compared with PCNA Index
- The Effect of 17beta- Estradiol on the Messenser Ribonucleic Acid ( mRNA ) Expression of Insulin-like Growth Factor Binding Proteins in Normal Myometrium and Uterine Leiomyoma
- Expression of estrogen receptor (ER)alpha, ERbeta and insulin-like growth factor binding protein related peptide-1 messenger ribonucleic acid in uterine leiomyoma and normal myometrium
- Expression of insulin-like growth factor [IGF] -Ia , IGF-Ib , and IGF-II messenger ribonucleic acid in uterine leiomyoma
- Insulin-Like Growth Factors and Their Bindign Proteins in Uterine Leiomyoma Pretreated with Gonadtropin Releasing Hormone Agonist