J Korean Med Sci.
2014 Oct;29(10):1416-1424. 10.3346/jkms.2014.29.10.1416.
Neural Substrates of Hanja (Logogram) and Hangul (Phonogram) Character Readings by Functional Magnetic Resonance Imaging
- Affiliations
-
- 1Neuroscience Research Institute, Gachon University, Incheon, Korea. neurokim@gachon.ac.kr
- 2Department of Psychology, Yeungnam University, Kyongsan, Korea.
- 3Kansei Fukushi Research Institute, Tohoku Fukushi University, Sendai, Japan.
- KMID: 2129634
- DOI: http://doi.org/10.3346/jkms.2014.29.10.1416
Abstract
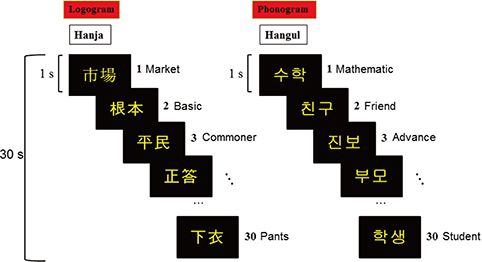
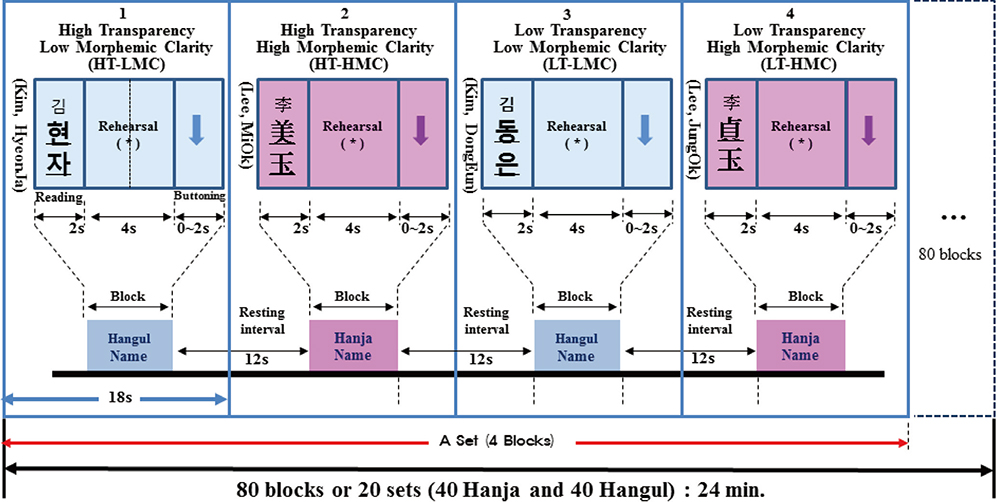
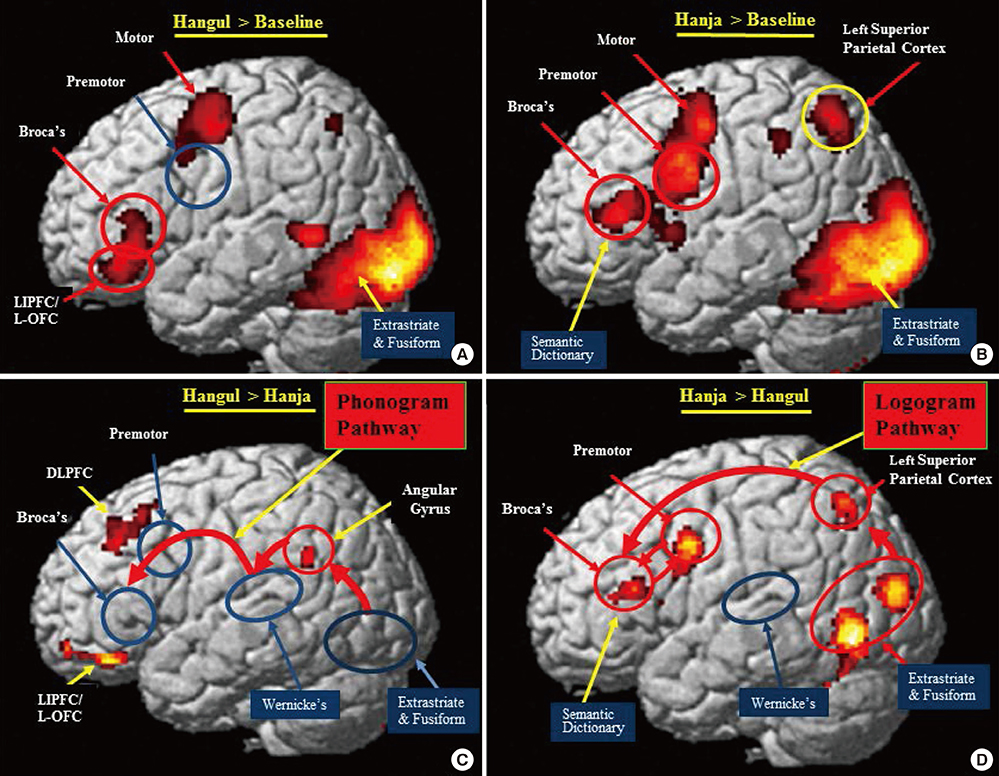
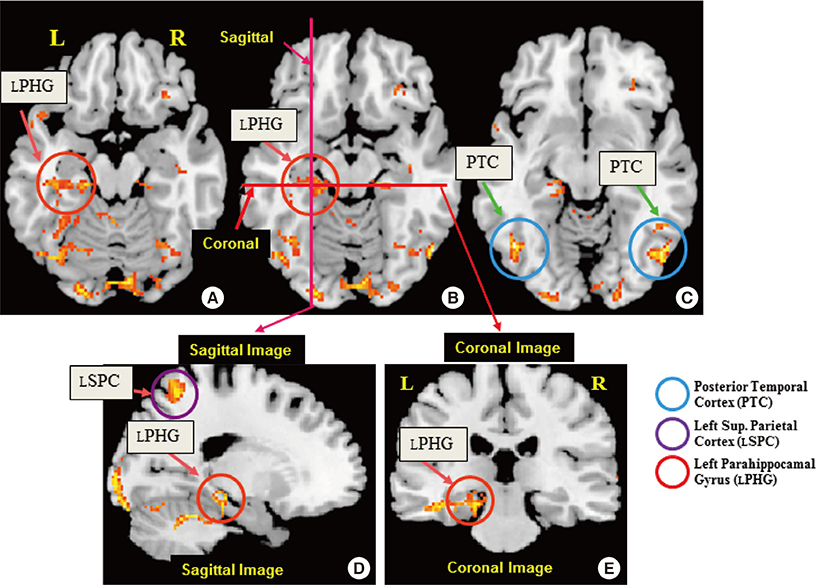
- The two basic scripts of the Korean writing system, Hanja (the logography of the traditional Korean character) and Hangul (the more newer Korean alphabet), have been used together since the 14th century. While Hanja character has its own morphemic base, Hangul being purely phonemic without morphemic base. These two, therefore, have substantially different outcomes as a language as well as different neural responses. Based on these linguistic differences between Hanja and Hangul, we have launched two studies; first was to find differences in cortical activation when it is stimulated by Hanja and Hangul reading to support the much discussed dual-route hypothesis of logographic and phonological routes in the brain by fMRI (Experiment 1). The second objective was to evaluate how Hanja and Hangul affect comprehension, therefore, recognition memory, specifically the effects of semantic transparency and morphemic clarity on memory consolidation and then related cortical activations, using functional magnetic resonance imaging (fMRI) (Experiment 2). The first fMRI experiment indicated relatively large areas of the brain are activated by Hanja reading compared to Hangul reading. The second experiment, the recognition memory study, revealed two findings, that is there is only a small difference in recognition memory for semantic transparency, while for the morphemic clarity was much larger between Hanja and Hangul. That is the morphemic clarity has significantly more effect than semantic transparency on recognition memory when studies by fMRI in correlation with behavioral study.
Keyword
MeSH Terms
Figure
Reference
-
1. Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: a meta-analysis. Hum Brain Mapp. 2005; 25:83–91.2. Lee KM. Functional MRI comparison between reading ideographic and phonographic scripts of one language. Brain Lang. 2004; 91:245–251.3. Libben G. Semantic transparency in the processing of compounds: consequences for representation, processing, and impairment. Brain Lang. 1998; 61:30–44.4. Amenta S, Crepaldi D. Morphological processing as we know it: an analytical review of morphological effects in visual word identification. Front Psychol. 2012; 3:232.5. Perfetti CA, Hart L. The lexical quality hypothesis. In : Verhoeven L, Carsten E, Reitsma P, editors. Precursors of functional literacy. Amsterdam; Philadelphia: John Benjamins Publishing Company;2002. p. 67–86.6. Perfetti C. Reading ability: lexical quality to comprehension. Sci Stud Read. 2007; 11:357–383.7. Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, et al. A cultural effect on brain function. Nat Neurosci. 2000; 3:91–96.8. Chen C, Xue G, Mei L, Chen C, Dong Q. Cultural neurolinguistics. Prog Brain Res. 2009; 178:159–171.9. Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010; 1191:62–88.10. Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992; 15:20–25.11. Tokunaga H, Nishikawa T, Ikejiri Y, Nakagawa Y, Yasuno F, Hashikawa K, Nishimura T, Sugita Y, Takeda M. Different neural substrates for Kanji and Kana writing: a PET study. Neuroreport. 1999; 10:3315–3319.12. Harpaz Y, Levkovitz Y, Lavidor M. Lexical ambiguity resolution in Wernicke's area and its right homologue. Cortex. 2009; 45:1097–1103.13. Nishitani N, Schürmann M, Amunts K, Hari R. Broca's region: from action to language. Physiology (Bethesda). 2005; 20:60–69.14. Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004; 92:67–99.15. Magnuson JS, Tanenhaus MK, Aslin RN, Dahan D. The time course of spoken word learning and recognition: studies with artificial lexicons. J Exp Psychol Gen. 2003; 132:202–227.16. Sakurai Y, Momose T, Iwata M, Sudo Y, Ohtomo K, Kanazawa I. Different cortical activity in reading of Kanji words, Kana words and Kana nonwords. Brain Res Cogn Brain Res. 2000; 9:111–115.17. Ino T, Tokumoto K, Usami K, Kimura T, Hashimoto Y, Fukuyama H. Longitudinal fMRI study of reading in a patient with letter-by-letter reading. Cortex. 2008; 44:773–781.18. Kwon M, Kim JS, Lee JH, Sim H, Nam K, Park H. Double dissociation of Hangul and Hanja reading in Korean patients with stroke. Eur Neurol. 2005; 54:199–203.19. Sakurai Y, Terao Y, Ichikawa Y, Ohtsu H, Momose T, Tsuji S, Mannen T. Pure alexia for kana. Characterization of alexia with lesions of the inferior occipital cortex. J Neurol Sci. 2008; 268:48–59.20. Sakurai Y, Asami M, Mannen T. Alexia and agraphia with lesions of the angular and supramarginal gyri: evidence for the disruption of sequential processing. J Neurol Sci. 2010; 288:25–33.21. Lucas TH 2nd, McKhann GM 2nd, Ojemann GA. Functional separation of languages in the bilingual brain: a comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients. J Neurosurg. 2004; 101:449–457.22. Usui K, Ikeda A, Takayama M, Matsuhashi M, Satow T, Begum T, Kinoshita M, Miyamoto S, Hashimoto N, Nagamine T, et al. Processing of Japanese morphogram and syllabogram in the left basal temporal area: electrical cortical stimulation studies. Brain Res Cogn Brain Res. 2005; 24:274–283.23. Gabrieli JD, Brewer JB, Poldrack RA. Images of medial temporal lobe functions in human learning and memory. Neurobiol Learn Mem. 1998; 70:275–283.24. Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999; 9:7–24.25. Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000; 20:6173–6180.26. Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001; 124:399–412.27. Carr VA, Viskontas IV, Engel SA, Knowlton BJ. Neural activity in the hippocampus and perirhinal cortex during encoding is associated with the durability of episodic memory. J Cogn Neurosci. 2010; 22:2652–2662.28. Diemand-Yauman C, Oppenheimer DM, Vaughan EB. Fortune favors the bold (and the Italicized): effects of disfluency on educational outcomes. Cognition. 2011; 118:111–115.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ideographic Alexia without Involvement of the Fusiform Gyrus in a Korean Stroke Patient: A Serial Functional Magnetic Resonance Imaging Study
- Dissociation of Hangul and Hanja Reading After Left Parieto-occipital Infarction: Alexia with agraphia with Hangul, but preserved in Hanja
- Hanja Alexia with Agraphia After Left Posterior Inferior Temporal Lobe Infarction: A Case Study
- Use of Korean Letter "Hangul" and Special Characters in a Chromosome Image Analyzer, QuipsTM 3.0 Operable Only in Mac OS 8.0
- Functional Magnetic Resonance Imaging of the Brain: Principle and Practical Application