Ann Lab Med.
2014 Nov;34(6):439-445. 10.3343/alm.2014.34.6.439.
Comparison of Supplemented Brucella Agar and Modified Clostridium difficile Agar for Antimicrobial Susceptibility Testing of Clostridium difficile
- Affiliations
-
- 1Department of Laboratory Medicine, Hanyang University College of Medicine, Seoul, Korea. jokang@hanyang.ac.kr
- 2Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2129569
- DOI: http://doi.org/10.3343/alm.2014.34.6.439
Abstract
- BACKGROUND
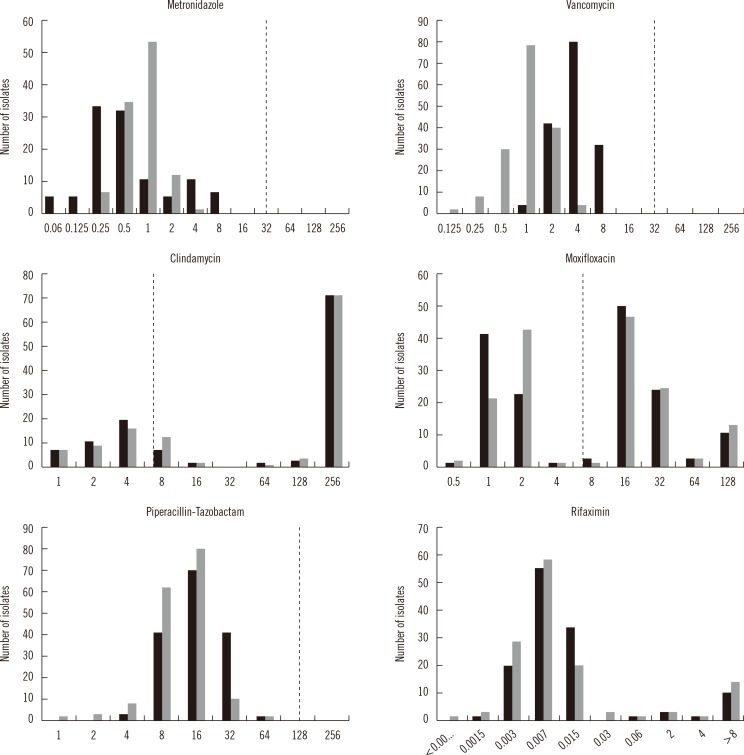
Antimicrobial susceptibility testing (AST) of Clostridium difficile is increasingly important because of the rise in resistant strains. The standard medium for the AST of C. difficile is supplemented Brucella agar (sBA), but we found that the growth of C. difficile on sBA was not optimal. Because active growth is critical for reliable AST, we developed a new, modified C. difficile (mCD) agar. C. difficile grew better on mCD agar than on sBA.
METHODS
C. difficile isolates were collected from patients with healthcare-associated diarrhea. sBA medium was prepared according to the CLSI guidelines. Homemade mCD agar containing taurocholate, L-cysteine hydrochloride, and 7% horse blood was used. For 171 C. difficile isolates, we compared the agar dilution AST results from mCD agar with those from sBA.
RESULTS
No significant differences were observed in the 50% minimal inhibitory concentration (MIC50) and 90% minimal inhibitory concentration (MIC90) of clindamycin (CLI), metronidazole (MTZ), moxifloxacin (MXF), piperacillin-tazobactam (PTZ), and rifaximin (RIX), but the values for vancomycin (VAN) were two-fold higher on mCD agar than on sBA. The MICs of CLI, MXF, and RIX were in 100% agreement within two-fold dilutions, but for MTZ, VAN, and PTZ, 13.7%, 0.6%, and 3.1% of the isolates, respectively, were outside the acceptable range.
CONCLUSIONS
The MIC ranges, MIC50 and MIC90, were acceptable when AST was performed on mCD agar. Thus, mCD agar could be used as a substitute medium for the AST of C. difficile.
Keyword
MeSH Terms
Figure
Reference
-
1. Marler LM, Siders JA, Wolters LC, Pettigrew Y, Skitt BL, Allen SD. Comparison of five cultural procedures for isolation of Clostridium difficile from stools. J Clin Microbiol. 1992; 30:514–516. PMID: 1537928.2. Arroyo LG, Rousseau J, Willey BM, Low DE, Staempfli H, McGeer A, et al. Use of a selective enrichment broth to recover Clostridium difficile from stool swabs stored under different conditions. J Clin Microbiol. 2005; 43:5341–5343. PMID: 16208013.3. Kelly CP, LaMont JT. Clostridium difficile-more difficult than ever. N Engl J Med. 2008; 359:1932–1940. PMID: 18971494.4. Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005; 173:1037–1042. PMID: 16179431.5. McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005; 353:2433–2441. PMID: 16322603.6. Peláez T, Alcalá L, Alonso R, Rodríguez-Créixems M, García-Lechuz JM, Bouza E. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother. 2002; 46:1647–1650. PMID: 12019070.7. Huang H, Weintraub A, Fang H, Nord CE. Antimicrobial resistance in Clostridium difficile. Int J Antimicrob Agents. 2009; 34:516–522. PMID: 19828299.8. Clinical and Laboratory Standards Institute. M11-A7. Methods for animicrobialsusceptibilitytesting of anaerobic bacteria; Approved Standard. 7th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2007.9. Bliss DZ, Johnson S, Clabots CR, Savik K, Gerding DN. Comparison of cycloserine-cefoxitin-fructose agar (CCFA) and taurocholate-CCFA for recovery of Clostridium difficile during surveillance of hospitalized patients. Diagn Microbiol Infect Dis. 1997; 29:1–4. PMID: 9350408.10. Aspinall ST, Hutchinson DN. New selective medium for isolating Clostridium difficile from faeces. J Clin Pathol. 1992; 45:812–814. PMID: 1401214.11. Erikstrup LT, Danielsen TK, Hall V, Olsen KE, Kristensen B, Kahlmeter G, et al. Antimicrobial susceptibility testing of Clostridium difficile using EUCAST epidemiological cut-off values and disk diffusion correlates. Clin Microbiol Infect. 2012; 18:E266–E272. PMID: 22672504.12. Bland JM. AltmanDG. Statistics notes-Transforming data. Br Med J. 1996; 312:770. PMID: 8605469.13. Davies BI. The importance of the geometric mean MIC. J Antimicrob Chemother. 1990; 25:471–472. PMID: 2338423.
Article14. Reynolds R, Hope R, Williams L. BSACWorking Parties on Resistance Surveillance. Survey, laboratory and statistical methods for the BSAC Resistance Surveillance Programmes. J Antimicrob Chemother. 2008; 62(S2):ii15–ii28. PMID: 18819976.15. Kim H, Jeong SH, Roh KH, Hong SG, Kim JW, Shin MG, et al. Investigation of toxin gene diversity, molecular epidemiology, and antimicrobial resistance of Clostridium difficile isolated from 12 hospitals in South Korea. Korean J Lab Med. 2010; 30:491–497. PMID: 20890081.16. Huang H, Wu S, Wang M, Zhang Y, Fang H, Palmgren AC, et al. Clostridium difficile infections in a Shanghai hospital: antimicrobial resistance, toxin profiles and ribotypes. Int J Antimicrob Agents. 2009; 33:339–342. PMID: 19097757.17. Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother. 2007; 51:2716–2719. PMID: 17517836.18. European Committee on Antimicrobial Susceptibility. Breakpoint tables for interpretation of MICs and zone diameters. Version 3.0. Basel: EUCAST;2013.19. Huang H, Weintraub A, Fang H, Wu S, Zhang Y, Nord CE. Antimicrobial susceptibility and heteroresistance in Chinese Clostridium difficile strains. Anaerobe. 2010; 16:633–635. PMID: 20849968.20. Chen J, Jiang XY, Chen XQ, Chen Y. Effect of temperature on the metronidazole-BSA interaction: Multi-spectroscopic method. J Mol Struct. 2008; 876:121–126.
Article21. Zietsman S, Kilian G, Worthington M, Stubbs C. Formulation development and stability studies of aqueous metronidazole benzoate suspensions containing various suspending agents. Drug Dev Ind Pharm. 2007; 33:191–197. PMID: 17454051.
Article22. Wang DP, Yeh MK. Degradation kinetics of metronidazole in solution. J Pharm Sci. 1993; 82:95–98. PMID: 8429500.
Article23. Kang JO, Han D, Choi TY. Evaluation of four methods for antimicrobial susceptibility testing of Helicobacter pylori in routine practice. Korean J Clin Microbiol. 2005; 8:82–89.24. Baines SD, O'Connor R, Freeman J, Fawley WN, Harmanus C, Mastrantonio P, et al. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother. 2008; 62:1046–1052. PMID: 18693234.25. Jiang ZD, DuPont HL, La Rocco M, Garey KW. In vitro susceptibility of Clostridium difficile to rifaximin and rifampin in 359 consecutive isolates at a university hospital in Houston, Texas. J Clin Pathol. 2010; 63:355–358. PMID: 20354207.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of ChromID Agar and Clostridium difficile Selective Agar for Effective Isolation of C. difficile from Stool Specimens
- Comparison of Clostridium difficile Toxin A Immunoassay with Cytotoxicity Assay
- Isolation and Identification of Clostridium difficile Using ChromID C. difficile Medium Combined With Gram Staining and PRO Disc Testing: A Proposal for a Simple Culture Process
- Evaluation of a ChromID C. difficile Agar for the Isolation of Clostridium difficile
- SDS-PAGE profiles of clostridium difficile isolated from patientsand hospital environments