J Korean Ophthalmol Soc.
2008 Feb;49(2):340-351. 10.3341/jkos.2008.49.2.340.
The Calretinin Immunoreactive Ganglion Cell Postsynaptic to the ON-Cholinergic Amacrine Cell in the Guinea Pig
- Affiliations
-
- 1Hangil Eye Hospital, Incheon, Korea.
- 2Department of Ophthalmology, College of Medicine, Ewha Womans University, Seoul, Korea. ckrey02@ewha.ac.kr
- 3Deparment of Ophthalmology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2127127
- DOI: http://doi.org/10.3341/jkos.2008.49.2.340
Abstract
-
PURPOSE: To demonstrate the characterization calretinin-immunoreactive displaced amacrine cells in the ganglion cell layer using immunohistochemistry and electron microscopy.
METHODS
For immunohistochemistry, sections from guinea pig retina were incubated with mouse monoclonal antibody directed against calretinin. For double label studies, sections were incuated in mixture of mouse monoclonal anti-calretinin or rabbit polyclonal anti-calretinin with following antibodies: goat polyclonal anti-ChAT, rabbit polyclonal anti-GABA, mouse monoclonal anti-GABAA receptor alpha1, beta2/3. Sections were analyzed using Bio-rad Radiance Plus confocal scanning microscope. Stained sections from three guinea pig were observed with transmission electron microscope.
RESULTS
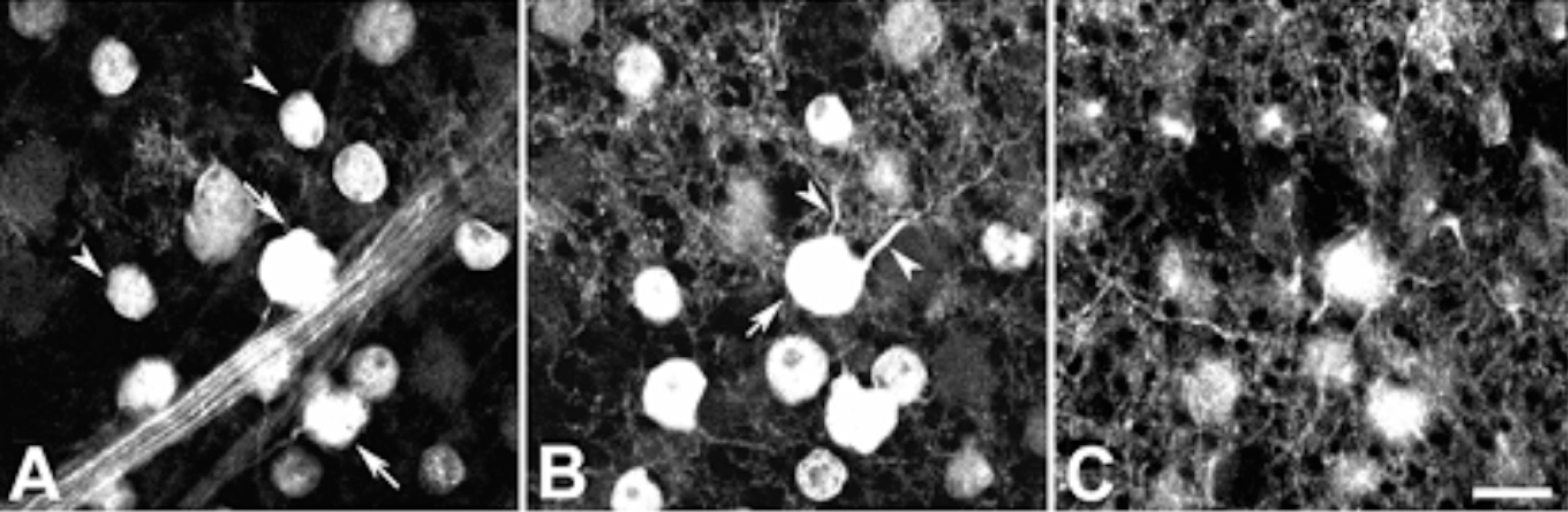
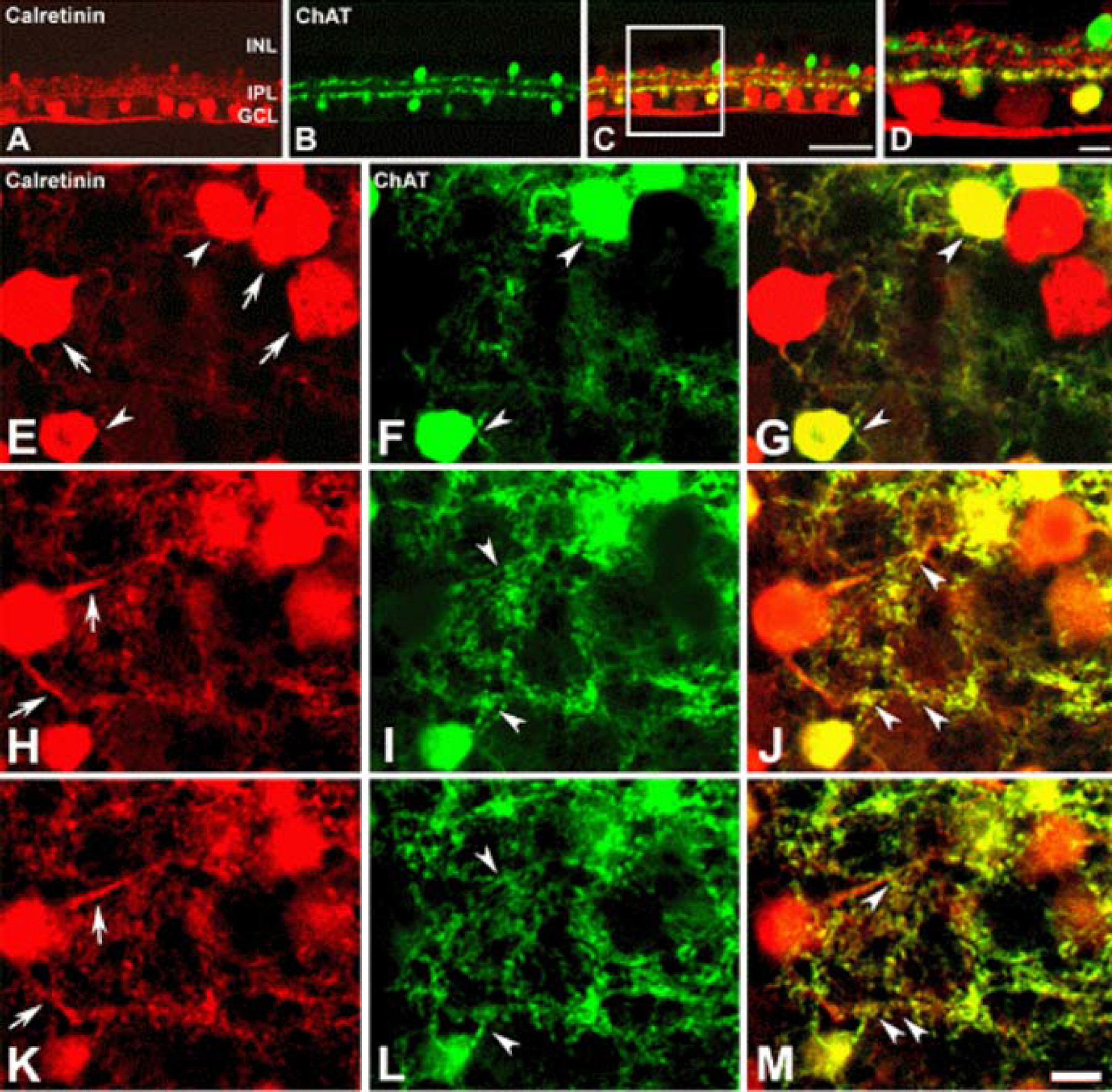
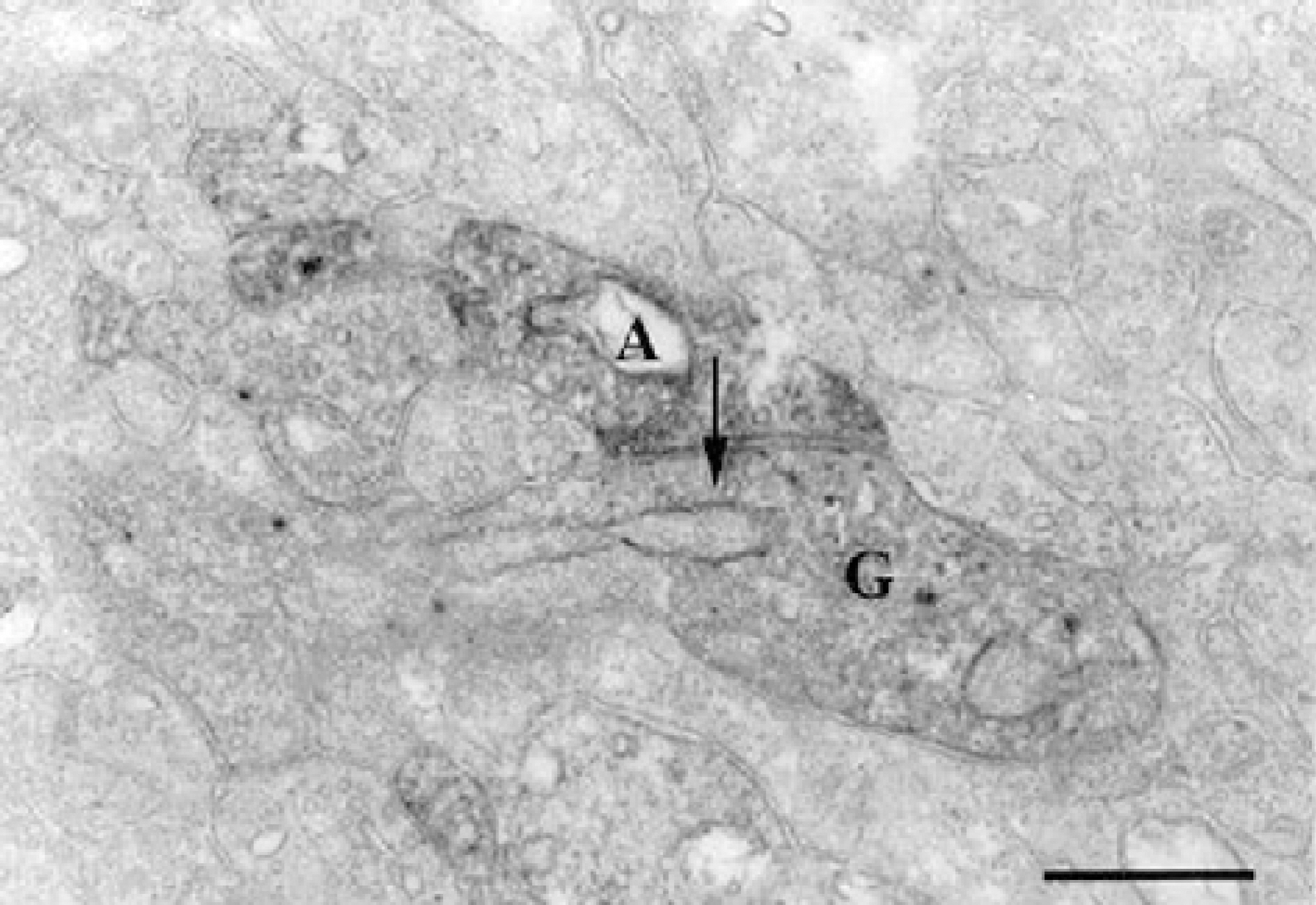
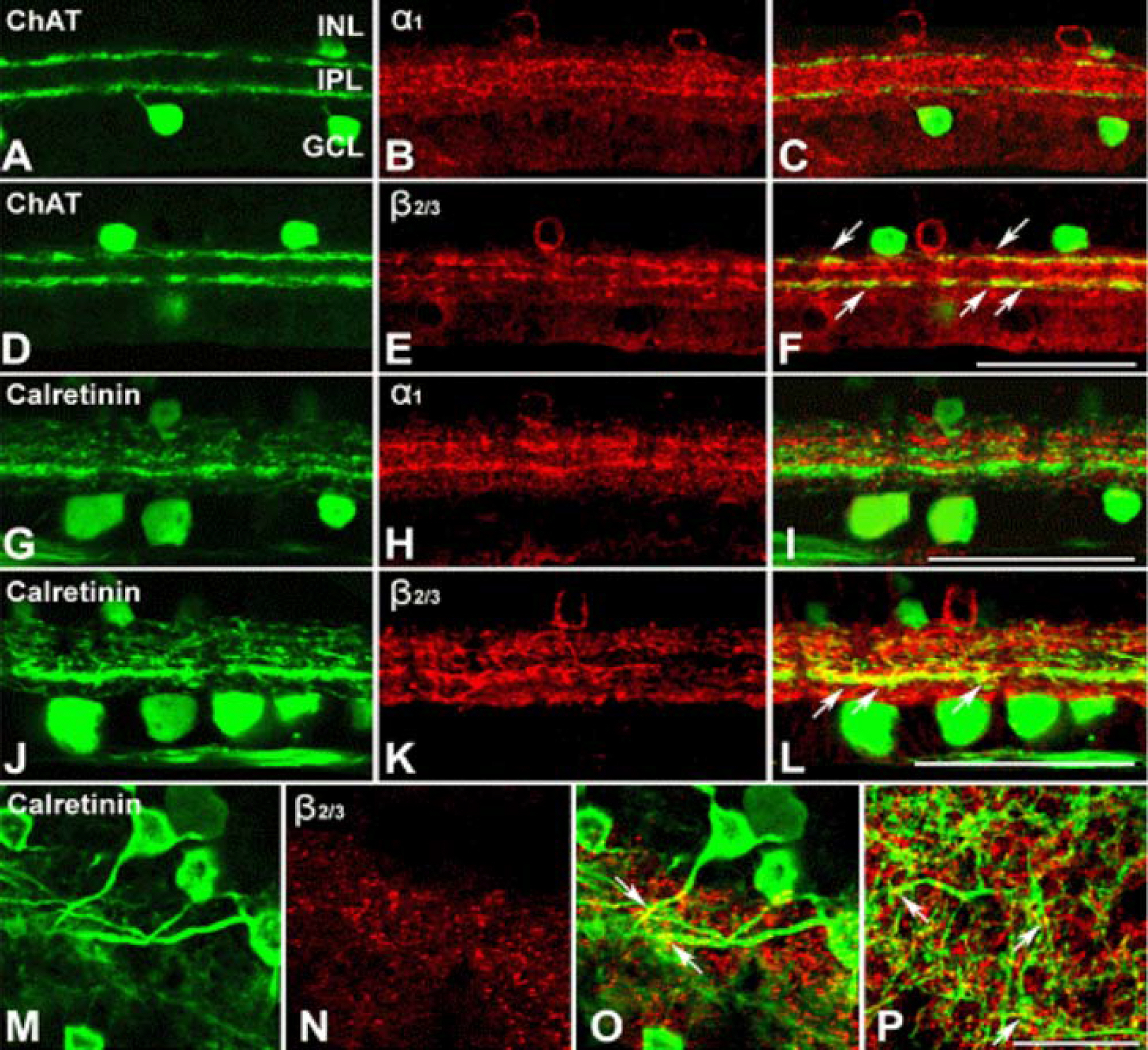
Calretinin immunoreactivity was present in displaced amacrine cells and ganglion cells gaving rise to processes ramified in the inner part of the inner plexiform layer in stratum 4. The same stratum was also occupied by the dendrites of ON-cholinergic amacrine cells. Double-labeling demonstrated that dendrites and cell bodies of displaced amacrine cells colocalized with ON-cholinergic amacrine cells and dendrites of ganglion cells directly overlapped with dendrites of ON-cholinergic amacrine cells. The synaptic connectivity was identified by electron microscopy. Ganglion cell dendrites received synaptic input from ON-cholinergic amacrine cell. GABAA receptor beta2/3 subunit bands cofaciculates the dendrites of displaced amacrine cell and ganglion cell that are juxtapose to the alpha1 subunit of GABAA receptor.
CONCLUSIONS
These results indicate that ON-cholinergic amacrine cells modulate calretinin-labeled ganglion cell via GABAA receptor beta2/3 in the guinea pig retina.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ikura M. Calcium binding and conformational response in EF-hand proteins. Trends Biochem Sci. 1996; 21:14–7.
Article2. Hamano K, Kiyama H, Emson PC, et al. Localization of two calcium binding proteins, calbindin (28kD) and parvalbumin (12 kD) in the vertebrate retina. J Comp Neurol. 1990; 302:417–24.3. Pasteels B, Rogers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci. 1990; 5:1–16.
Article4. Goebel DJ, Pourcho RG. Calretinin in the cat retina: colocalizations with other calcium-binding proteins, GABA and glycine. Vis Neurosci. 1997; 14:311–22.
Article5. Wässle H, Grüert U, Rörenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993; 332:407–20.6. Casini G, Rickman DW, Brecha NC. AII amacrine cell population in the rabbit retina: identification by parvalbumin immunoreactivity. J Comp Neurol. 1995; 356:132–42.
Article7. Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996; 366:15–33.
Article8. Famiglietti EV Jr. ‘Starburst’ amacrine cells and cholinergic neurons: mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res. 1983; 261:138–44.
Article9. Gabriel R, Witkovsky P. Cholinergic, but not the rod pathway-related glycinergic(All), amacrine cells contain calretinin in the rat retina. Neurosci Lett. 1998; 247:179–82.10. Volgyi B, Pollak E, Buzas P, et al. Calretinin in neurochemically well-defined cell populations of rabbit retina. Brain Res. 1997; 763:79–86.11. Araki CM, Hamassaki-Britto DE. Calretinin co-localizes with the NMDA receptor subunit NR1 in cholinergic amacrine cells of the rat retina. Brain Res. 2000; 869:220–4.
Article12. Brandon C. Cholinergic neurons in the rabbit retina: dendritic branching and ultrastructural connectivity. Brain Res. 1987; 426:119–30.
Article13. Mariani AP, Hersh LB. Synaptic organization of cholinergic amacrine cells in the rhesus monkey retina. J Comp Neurol. 1988; 267:269–80.
Article14. Famiglietti EV Jr. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J Comp Neurol. 1991; 309:40–70.
Article15. Masland RH, Ames A. Responses to acetylcholine of ganglion cells in an isolated mammalian retina. J Neurophysiol. 1976; 39:1220–35.
Article16. Schmidt M, Humphrey MF, Wässle H. Action and localization of acetylcholine in the cat retina. J Neurophysiol. 1987; 58:997–1015.
Article17. Lipton SA, Aizenman E, Loring RH. Neural nicotinic acetylcholine responses in solitary mammalian retinal ganglion cells. Pflugers Arch. 1987; 410:37–43.
Article18. Downing JE, Kaneko A. Cat retinal ganglion cells show transient responses to acetylcholine and sustained responses to Lglutamate. Neurosci Lett. 1992; 137:114–8.
Article19. Kaneda M, Hashimoto M, Kaneko A. Neuronal nicotinic acetylcholine receptors of ganglion cells in the cat retina.
Article20. Araki CM, Pires RS, Britto LR, et al. Differential co-localization of nicotinic acetylcholine receptor subunits with calcium-binding proteins in retinal ganglion cells. Brain Res. 1997; 774:250–5.
Article21. Ariel M, Daw NW. Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J Physiol. 1982; 324:161–85.
Article22. Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol. 1997; 77:675–89.
Article23. Daw NW, Wyatt HJ. Kittens reared in a unidirectional environment: evidence for a critical period. J Physiol. 1976; 257:155–70.
Article24. Famiglietti EV Jr. Dendritic co-stratification of ON and ON-OFF directionally selective ganglion cells with starburst amacrine cells in rabbit retina. J Comp Neurol. 1992; 324:322–35.
Article25. He S, Masland RH. ON direction-selective ganglion cells in the rabbit retina: dendritic morphology and pattern of fasciculation. Vis Neurosci. 1998; 15:369–75.
Article26. Famiglietti EV Jr, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975; 84:293–300.
Article27. Vaney DI. The morphology and topographic distribution of AII amacrine cells in the cat retina. Proc R Soc Lond B Biol Sci. 1985; 224:475–88.28. Dacheux RF, Raviola E. The rod pathway in the rabbit retina: A depolarizing bipolar and amacrine cell. J Neurosci. 1986; 6:331–45.
Article29. Wong RO, Henry GH, Medveczky CJ. Bistratified amacrine cells in the retina of the Tammar wallaby Macropus eugenii. Exp Brain Res. 1986; 63:102–5.
Article30. Mills SL, Massey SC. Labeling and distribution of AII amacrine cells in the rabbit retina. J Neurol. 1991; 304:491–501.
Article31. Vaney DI, Gynther IC, Young HM. Rod-signal interneurons in the rabbit retina: 2. AII amacrine cells. J Comp Neurol. 1991; 310:154–69.
Article32. Schmidt M, Wassle H, Humphrey M. Number and distribution of putative cholinergic neurons in the cat retina. Neurosci Lett. 1985; 59:235–40.
Article33. Wässle H, Peichl L, Boycott BB. Mosaics and territories of cat retinal ganglion cells. Prog Brain Res. 1983; 58:183–90.
Article34. Pourcho RG, Osman K. Acetylcholinesterase localization in cat retina: a comparison with choline acetyltransferase. Exp Eye Res. 1986; 43:585–94.
Article35. Rice DS, Curran T. Disabled-1 is expressed in type AII amacrine cells in the mouse retina. J Comp Neurol. 2000; 424:327–38.
Article36. Voigt T. Cholinergic amacrine cells in the rat retina. J Comp Neurol. 1986; 248:19–35.
Article37. Lee EJ, Kim HJ, Kim IB, et al. Morphological analysis of Disabled-1-immunoreactive amacrine cells in the guinea pig retina. J Comp Neurol. 2003; 466:240–50.
Article38. Famiglietti EV Jr, Tumosa N. Immunocytochemical staining of cholinergic amacrine cells in rabbit retina. Brain Res. 1987; 413:398–403.
Article39. Mariani AP, Cosenza-Murphy D, Barker JL. GABAergic synapses and benzodiazepine receptors are not identically distributed in the primate retina. Brain Res. 1987; 415:153–7.
Article40. Mitrofanis J, Maslim J, Stone J. Catecholaminergic and cholinergic neurons in the developing retina of the rat. J Comp Neurol. 1988; 276:343–59.
Article41. Rodieck RW, Marshak DW. Spatial density and distribution of choline acetyltransferase immunoreactive cells in human, macaque, and baboon retinas. J Comp Neurol. 1992; 321:46–64.
Article42. Kim IB, Lee EJ, Kim MK, et al. Choline acetyltransferase-immunoreactive neurons in the developing rat retina. J Comp Neurol. 2000; 427:604–16.
Article43. Vardi N, Masarachia PJ, Sterling P. Structure of the starburst amacrine network in the cat retina and its association with alpha ganglion cells. J Comp Neurol. 1989; 288:601–11.
Article44. Gabriel R, Volgyi B, Pollak E. Most calretinin-containing amacrine cells in the rabbit retina co-localize glycine. Vis Neurosci. 1999; 16:983–90.
Article45. Kosaka T, Tauchi M, Dahl JL. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunore-activities in various brain regions of the rat. Exp Brain Res. 1988; 70:605–17.
Article46. Brecha N, Johnson D, Peichl L, Wassle H. Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and gamma-aminobutyrate immunoreactivity. Proc Natl Acad Sci U S A. 1988; 85:6187–91.
Article47. Richards JG, Schoch P, Haring P, et al. Resolving GABA A/benzodiazepine receptors: cellular and subcellular localization in the CNS with monoclonal antibodies. J Neurosci. 1987; 7:1866–86.48. Hughes TE, Carey RG, Vitorica J, et al. Immunohistochemical localization of GABA A receptors in the retina of the new world primate Saimiri sciureus. Vis Neurosci. 1989; 2:565–81.49. Greferath U, Muller F, Wassle H, et al. Localization of GABA A receptors in the rat retina. Vis Neurosci. 1993; 10:551–61.50. Grunert U, Greferath U, Boycott BB, Wassle H. Parasol (P alpha) ganglion-cells of the primate fovea: immunocytochemical staining with antibodies against GABA A-receptors. Vision Res. 1993; 33:1–14.51. Gruert U, Hughes TE. Immunohistochemical localization of GABA A receptors in the scotopic pathway of the cat retina. Cell Tissue Res. 1993; 274:267–77.52. Zucker CL, Ehinger B. Gamma-aminobutyric acid A receptors on a bistratified amacrine cell type in the rabbit retina. J Comp Neurol. 1998; 393:309–19.53. Brandstatter JH, Greferath U, Euler T, Wassle H. Co-stratification of GABA A receptors with the directionally selective circuitry of the rat retina. Vis Neurosci. 1995; 12:345–58.54. Wässle H, Riemann HJ. The mosaic of nerve cells in the mammalian retina. Proc R Soc Lond B Biol Sci. 1978; 200:441–61.55. Wässle H, Peichl L, Boycott BB. Dendritic territories of cat retinal ganglion cells. Nature. 1981; 292:344–5.
Article56. Rockhill RL, Euler T, Masland RH. Spatial order within but not between types of retinal neurons. Proc Natl Acad Sci U S A. 2000; 97:2303–7.
Article57. Famiglietti EV Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976; 194:193–5.
Article58. Nelson R, Famiglietti EV, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978; 41:472–83.
Article59. Famiglietti EV. Starburst amacrine cells: morphological constancy and systematic variation in the anisotropic field of rabbit retinal neurons. J Neurosci. 1985; 5:562–77.
Article60. Bloomfield SA. Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. J Neurophysiol. 1992; 68:711–25.
Article61. Taylor WR, Wässle H. Receptive field properties of starburst cholinergic amacrine cells in the rabbit retina. Eur J Neurosci. 1995; 7:2308–21.
Article62. Peters BN, Masland RH. Responses to light of starburst amacrine cells. J Neurophysiol. 1996; 75:469–80.
Article63. Yamada ES, Dmitrieva N, Keyser KT, et al. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J Comp Neurol. 2003; 461:76–90.
Article64. Vardi N, Masarachia P, Sterling P. Immunoreactivity to GABA A receptor in the outer plexiform layer of the cat retina. J Comp Neurol. 1992; 320:394–7.65. Jacoby R, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. J Neurosci. 1996; 16:8041–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution Pattern and Synaptic Circuitry of Cholinergic Neurons in the Rat Retina
- Immunocytochemical Localization of GABA Transporter-3 in the Guinea Pig Retina
- Immunocytochemical Study on Synaptic Circuitry of Glycinergic Neurons in the Rat Retina
- Colocalization of GABA and Glycine within the Neurons of the Rat Retina
- Changes in transcript and protein levels of calbindin D28k, calretinin and parvalbumin, and numbers of neuronal populations expressing these proteins in an ischemia model of rat retina