J Korean Soc Radiol.
2015 Jun;72(6):393-400. 10.3348/jksr.2015.72.6.393.
Correlation of Magnetic Resonance Imaging Findings of Spinal Intradural Extramedullary Schwannomas with Pathologic Findings
- Affiliations
-
- 1Department of Radiology, Inha University Hospital, Incheon, Korea.
- 2Department of Pathology, Inha University Hospital, Incheon, Korea. radzzang@gmail.com
- 3Department of Neurosurgery, Inha University Hospital, Incheon, Korea.
- KMID: 2098018
- DOI: http://doi.org/10.3348/jksr.2015.72.6.393
Abstract
- PURPOSE
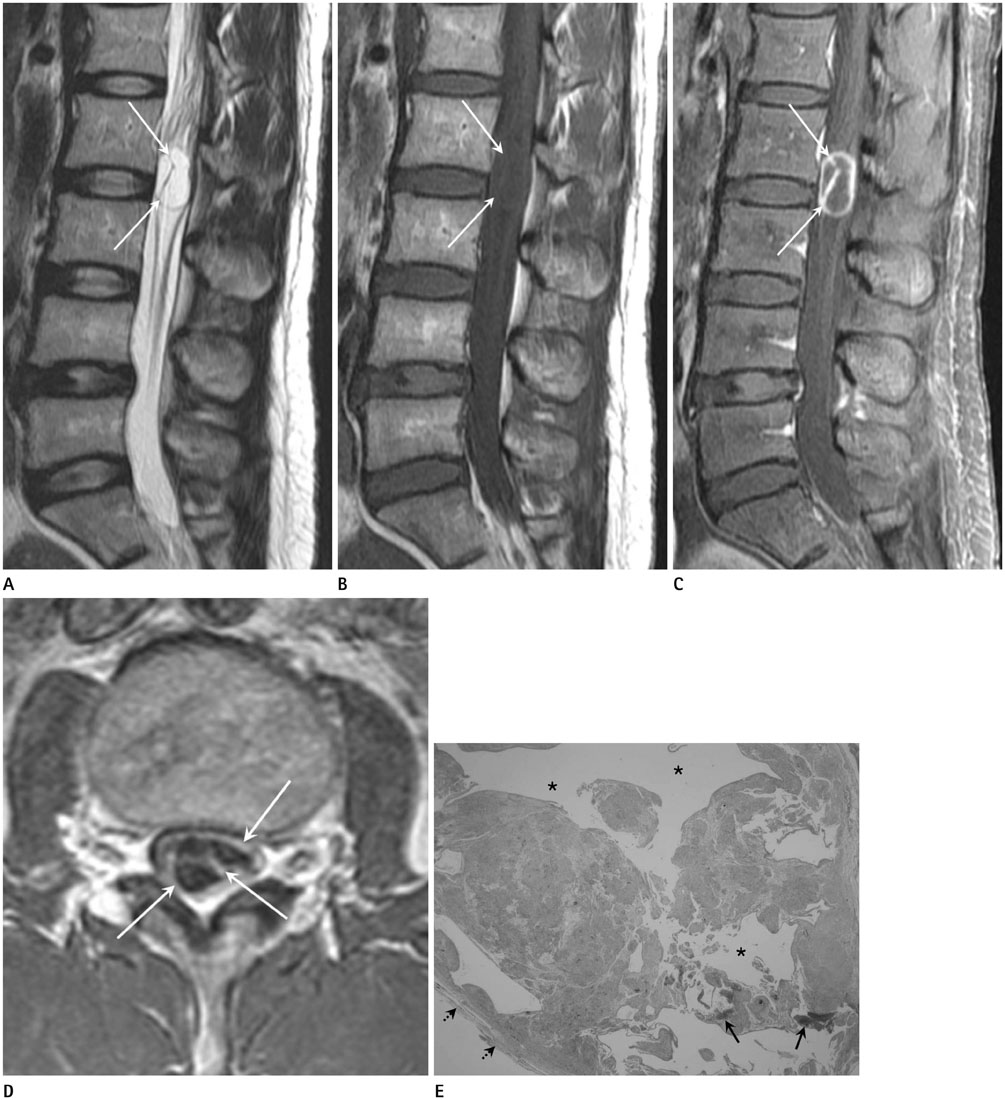
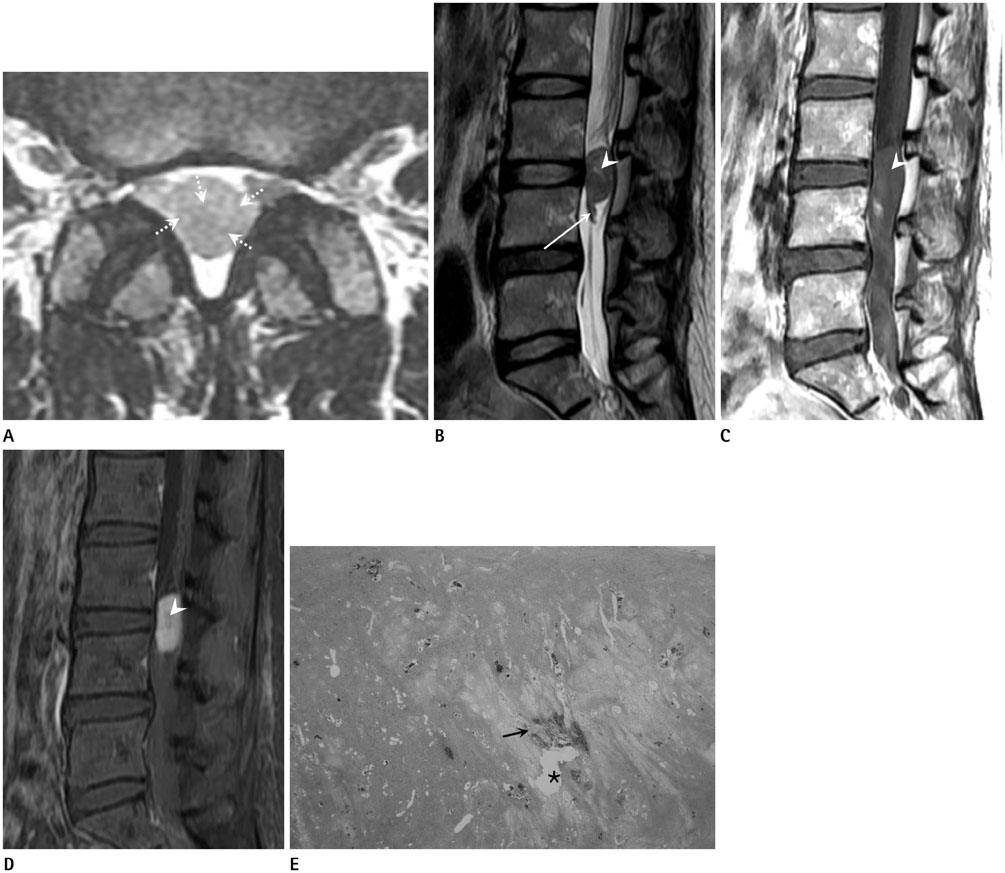
To evaluate the magnetic resonance imaging (MRI) findings of spinal intradural extramedullary schwannomas with pathologic correlation and to determine whether these schwannomas share the imaging features of schwannomas in the peripheral nerves.
MATERIALS AND METHODS
The MRIs of 17 cases of pathologically proven spinal intradural extramedullary schwannomas were reviewed retrospectively, and cystic changes, enhancement, and intratumoral hemorrhage of the tumors were evaluated. Imaging features known to be common findings of schwannoma in the peripheral nerves, such as encapsulation, the target sign, the fascicular sign, and visualization of entering or exiting nerve rootlets, were also evaluated. The histopathology of the tumors was correlated with the MRI findings.
RESULTS
Cystic changes were detected in 14 cases by MRI and in 16 cases by pathology. The most common pattern of enhancement was a thick peripheral septal pattern (70.59%). Intratumoral hemorrhage was detected in four cases on MRI, but in all cases on pathology. Encapsulation was observed in all cases. The fascicular sign was seen in only four cases, and thickening of an exiting rootlet was visualized in one case. None of the cases showed the target sign.
CONCLUSION
Spinal intradural extramedullary schwannomas were typical encapsulated cystic tumors and had few imaging features of schwannomas in the peripheral nerves.
MeSH Terms
Figure
Reference
-
1. Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple HT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999; 19:1253–1280.2. Jee WH, Oh SN, McCauley T, Ryu KN, Suh JS, Lee JH, et al. Extraaxial neurofibromas versus neurilemmomas: discrimination with MRI. AJR Am J Roentgenol. 2004; 183:629–633.3. Isobe K, Shimizu T, Akahane T, Kato H. Imaging of ancient schwannoma. AJR Am J Roentgenol. 2004; 183:331–336.4. Friedman DP, Tartaglino LM, Flanders AE. Intradural schwannomas of the spine: MR findings with emphasis on contrast-enhancement characteristics. AJR Am J Roentgenol. 1992; 158:1347–1350.5. Sze G, Bravo S, Krol G. Spinal lesions: quantitative and qualitative temporal evolution of gadopentetate dimeglumine enhancement in MR imaging. Radiology. 1989; 170(3 Pt 1):849–856.6. Demachi H, Takashima T, Kadoya M, Suzuki M, Konishi H, Tomita K, et al. MR imaging of spinal neurinomas with pathological correlation. J Comput Assist Tomogr. 1990; 14:250–254.7. Borges G, Bonilha L, Proa M Jr, Fernandes YB, Ramina R, Zanardi V, et al. Imaging features and treatment of an intradural lumbar cystic schwannoma. Arq Neuropsiquiatr. 2005; 63:681–684.8. Koga H, Matsumoto S, Manabe J, Tanizawa T, Kawaguchi N. Definition of the target sign and its use for the diagnosis of schwannomas. Clin Orthop Relat Res. 2007; 464:224–229.9. Kransdorf M, Murphey MD. Neurogenic tumors. In : Kransdorf MJ, Murphey MD, editors. Imaging of soft tissue tumors. Philadelphia, PA: Saunders;1997. p. 235–273.10. Katonis P, Kontakis G, Pasku D, Tzermiadianos M, Tzanakakis G, Hadjipavlou A. Intradural tumours of the lumbar spine presenting with low back pain: report of two cases and review of the literature. Acta Orthop Belg. 2008; 74:282–288.11. Conti P, Pansini G, Mouchaty H, Capuano C, Conti R. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004; 61:34–43. discussion 44.12. De Verdelhan O, Haegelen C, Carsin-Nicol B, Riffaud L, Amlashi SF, Brassier G, et al. MR imaging features of spinal schwannomas and meningiomas. J Neuroradiol. 2005; 32:42–49.13. Van Goethem JW, van den, Ozsarlak O, De Schepper AM, Parizel PM. Spinal tumors. Eur J Radiol. 2004; 50:159–176.14. Mhatre P, Hudgins PA, Hunter S. Dermoid cyst in the lumbosacral region: radiographic findings. AJR Am J Roentgenol. 2000; 174:874–875.15. Thompson DN. Spinal inclusion cysts. Childs Nerv Syst. 2013; 29:1647–1655.16. Teksam M, Casey SO, Michel E, Benson M, Truwit CL. Intraspinal epidermoid cyst: diffusion-weighted MRI. Neuroradiology. 2001; 43:572–574.17. Matsui H, Kanamori M, Yudoh K, Ohmori K, Yasuda T, Wakaki K. Cystic spinal cord tumors: magnetic resonance imaging correlated to histopathological findings. Neurosurg Rev. 1998; 21:147–151.18. Leite CC, Jinkins JR, Escobar BE, Magalhães AC, Gomes GC, Dib G, et al. MR imaging of intramedullary and intradural-extramedullary spinal cysticercosis. AJR Am J Roentgenol. 1997; 169:1713–1717.19. Güneçs M, Akdemir H, TugXMLLink_XYZcu B, Günaldi O, Gümüçs E, Akpinar A. Multiple intradural spinal hydatid disease: a case report and review of literature. Spine (Phila Pa 1976). 2009; 34:E346–E350.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Total Excision of a Giant Ventral Midline Cervical Spinal Intradural Schwannoma via Posterior Approach
- MR Imaging of Intradural Extramedullary Tuberculoma of the Spinal Cord: Report of Two Cases
- Non-Enhancing Intradural Extramedullary Ependymoma: A Case Report
- A Case of Intradural, Extramedullary Tuberculous Granuloma Developed During the Treatment of Tuberculous Meningitis
- Ventral Schwannoma of the Thoracolumbar Spine