J Periodontal Implant Sci.
2012 Jun;42(3):88-94. 10.5051/jpis.2012.42.3.88.
Comparative evaluation of roughness of titanium surfaces treated by different hygiene instruments
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- KMID: 2094732
- DOI: http://doi.org/10.5051/jpis.2012.42.3.88
Abstract
- PURPOSE
The use of appropriate instruments to clean surfaces with minimal change, is critical for the successful maintenance of a dental implant. However, there is no consensus about the type and methodology for such instruments. The aim of this study was to characterize changes in the roughness of titanium surfaces treated by various scaling instruments.
METHODS
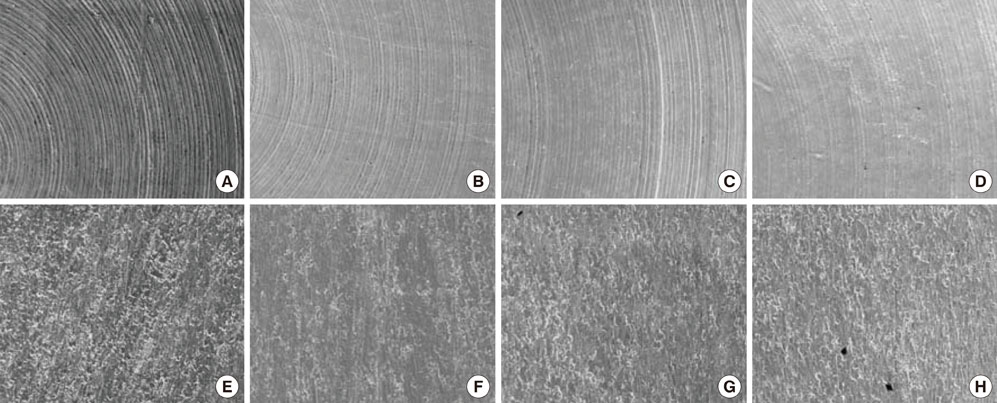
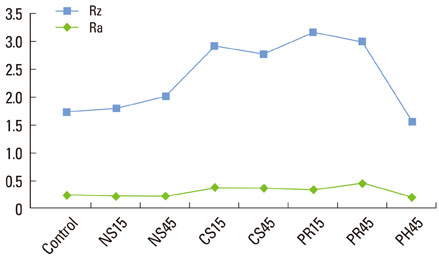
Thirty-seven identical disks (5 mm in diameter) were investigated in this study. The specimens were divided into eight groups according to the types of instrumentation and the angle of application. Ultrasonic scaling systems were applied on a titanium disk to simulate standard clinical conditions. The equipment included a piezoelectric ultrasonic scaler with a newly developed metallic tip (NS group), a piezoelectric ultrasonic scaler with a conventional tip (CS group), a piezoelectric root planer ultrasonic scaler with a conventional tip (PR group), and a plastic hand curette (PH group). In addition, the sites treated using piezoelectric ultrasonic scaler systems were divided two sub-groups: 15 and 45 degrees. The treated titanium surfaces were observed by scanning electron microscopy (SEM), and the average surface roughness (Ra) and mean roughness profile depth (Rz) were measured with a profilometer.
RESULTS
SEM no significant changes in the titanium surfaces in the NS group, regardless of the angle of application. The PH group also showed no marked changes to the titanium surface, although some smoothening was observed. All CS and PR sites lost their original texture and showed irregular surfaces in SEM analysis. The profilometer analysis demonstrated that the roughness values (Ra and Rz) of the titanium surfaces increased in all, except the PH and NS groups, which showed roughness decreases relative to the untreated control group. The Ra value differed significantly between the NS and PR groups (P<0.05).
CONCLUSIONS
The results of this study indicated that changes in or damage to titanium surfaces might be more affected by the hardness of the scaler tip than by the application method. Within the limitations of this study, the newly developed metallic scaler tip might be especially suitable for peri-implant surface decontamination, due to its limited effects on the titanium surface.
MeSH Terms
Figure
Cited by 1 articles
-
Decontamination methods to restore the biocompatibility of contaminated titanium surfaces
Seong-Ho Jin, Eun-Mi Lee, Jun-Beom Park, Kack-Kyun Kim, Youngkyung Ko
J Periodontal Implant Sci. 2019;49(3):193-204. doi: 10.5051/jpis.2019.49.3.193.
Reference
-
1. Lindhe J, Meyle J. Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008. 35:8 Suppl. 282–285.
Article2. Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987. 2:145–151.
Article3. Leonhardt A, Renvert S, Dahlen G. Microbial findings at failing implants. Clin Oral Implants Res. 1999. 10:339–345.
Article4. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999. 284:1318–1322.
Article5. Lamont RJ, Jenkinson HF. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immunol. 2000. 15:341–349.
Article6. Fransson C, Lekholm U, Jemt T, Berglundh T. Prevalence of subjects with progressive bone loss at implants. Clin Oral Implants Res. 2005. 16:440–446.
Article7. Renvert S, Persson GR. Periodontitis as a potential risk factor for peri-implantitis. J Clin Periodontol. 2009. 36:Suppl 10. 9–14.
Article8. Renvert S, Roos-Jansaker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008. 35:8 Suppl. 305–315.
Article9. Lang NP, Berglundh T. Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: where are we now? Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011. 38:Suppl 11. 178–181.
Article10. Leonhardt A, Dahlen G, Renvert S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol. 2003. 74:1415–1422.
Article11. Augthun M, Tinschert J, Huber A. In vitro studies on the effect of cleaning methods on different implant surfaces. J Periodontol. 1998. 69:857–864.
Article12. Thomson-Neal D, Evans GH, Meffert RM. Effects of various prophylactic treatments on titanium, sapphire, and hydroxyapatite-coated implants: an SEM study. Int J Periodontics Restorative Dent. 1989. 9:300–311.13. Barnes CM, Fleming LS, Mueninghoff LA. SEM evaluation of the in-vitro effects of an air-abrasive system on various implant surfaces. Int J Oral Maxillofac Implants. 1991. 6:463–469.14. Cross-Poline GN, Shaklee RL, Stach DJ. Effect of implant curets on titanium implant surfaces. Am J Dent. 1997. 10:41–45.15. Hallmon WW, Waldrop TC, Meffert RM, Wade BW. A comparative study of the effects of metallic, nonmetallic, and sonic instrumentation on titanium abutment surfaces. Int J Oral Maxillofac Implants. 1996. 11:96–100.
Article16. Sato S, Kishida M, Ito K. The comparative effect of ultrasonic scalers on titanium surfaces: an in vitro study. J Periodontol. 2004. 75:1269–1273.
Article17. Kawashima H, Sato S, Kishida M, Ito K. A comparison of root surface instrumentation using two piezoelectric ultrasonic scalers and a hand scaler in vivo. J Periodontal Res. 2007. 42:90–95.
Article18. Schwarz F, Rothamel D, Sculean A, Georg T, Scherbaum W, Becker J. Effects of an Er:YAG laser and the Vector ultrasonic system on the biocompatibility of titanium implants in cultures of human osteoblast-like cells. Clin Oral Implants Res. 2003. 14:784–792.
Article19. Schwarz F, Papanicolau P, Rothamel D, Beck B, Herten M, Becker J. Influence of plaque biofilm removal on reestablishment of the biocompatibility of contaminated titanium surfaces. J Biomed Mater Res A. 2006. 77:437–444.
Article20. Schwarz F, Ferrari D, Popovski K, Hartig B, Becker J. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J Biomed Mater Res B Appl Biomater. 2009. 88:83–91.
Article21. Berglundh T, Lindhe J, Marinello C, Ericsson I, Liljenberg B. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin Oral Implants Res. 1992. 3:1–8.
Article22. Salonen MA, Oikarinen K, Virtanen K, Pernu H. Failures in the osseointegration of endosseous implants. Int J Oral Maxillofac Implants. 1993. 8:92–97.
Article23. Kwan JY, Zablotsky MH, Meffert RM. Implant maintenance using a modified ultrasonic instrument. J Dent Hyg. 1990. 64:422424–425. 43024. Fox SC, Moriarty JD, Kusy RP. The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol. 1990. 61:485–490.
Article25. Rapley JW, Swan RH, Hallmon WW, Mills MP. The surface characteristics produced by various oral hygiene instruments and materials on titanium implant abutments. Int J Oral Maxillofac Implants. 1990. 5:47–52.26. Gantes BG, Nilveus R. The effects of different hygiene instruments on titanium surfaces: SEM observations. Int J Periodontics Restorative Dent. 1991. 11:225–239.27. Quirynen M, van der Mei HC, Bollen CM, Schotte A, Marechal M, Doornbusch GI, et al. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J Dent Res. 1993. 72:1304–1309.
Article28. Mengel R, Meer C, Flores-de-Jacoby L. The treatment of uncoated and titanium nitride-coated abutments with different instruments. Int J Oral Maxillofac Implants. 2004. 19:232–238.29. Bailey GM, Gardner JS, Day MH, Kovanda BJ. Implant surface alterations from a nonmetallic ultrasonic tip. J West Soc Periodontol Periodontal Abstr. 1998. 46:69–73.30. Ruhling A, Kocher T, Kreusch J, Plagmann HC. Treatment of subgingival implant surfaces with Teflon-coated sonic and ultrasonic scaler tips and various implant curettes. An in vitro study. Clin Oral Implants Res. 1994. 5:19–29.
Article31. Parham PL Jr, Cobb CM, French AA, Love JW, Drisko CL, Killoy WJ. Effects of an air-powder abrasive system on plasma-sprayed titanium implant surfaces: an in vitro evaluation. J Oral Implantol. 1989. 15:78–86.32. Van de Velde E, Thielens P, Schautteet H, Vanclooster R. Subcutaneous emphysema of the oral floor during cleaning of a bridge fixed on an IMZ implant. Case report. Rev Belge Med Dent (1984). 1991. 46:64–71.33. Davis JR. Metals handbook: desk edition. 1998. 2nd ed. Materials Park: ASM International;1510–1511.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Root surface roughness following mechanical instrumentation, in vitro 3 dimensional planimetric study
- Influence of scaling procedures on the integrity of titanium nitride coated CAD/CAM abutments
- Attachment of Human Gingival Fibroblasts to Commercially Pure Titanium Surfaces with Different Instruments: A comparative Study in Vitro
- The Effects of Citric Acid on HA coated Implant Surface
- Effect of a metallic ultrasonic scaler tip on titanium surfaces: a preliminary study