Korean J Physiol Pharmacol.
2008 Feb;12(1):13-23. 10.4196/kjpp.2008.12.1.13.
Roles of Dopaminergic D1 and D2 Receptors in Catecholamine Release from the Rat Adrenal Medulla
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Chosun University, Gwangju 501-759, Korea. dylim@chosun.ac.kr
- KMID: 2071641
- DOI: http://doi.org/10.4196/kjpp.2008.12.1.13
Abstract
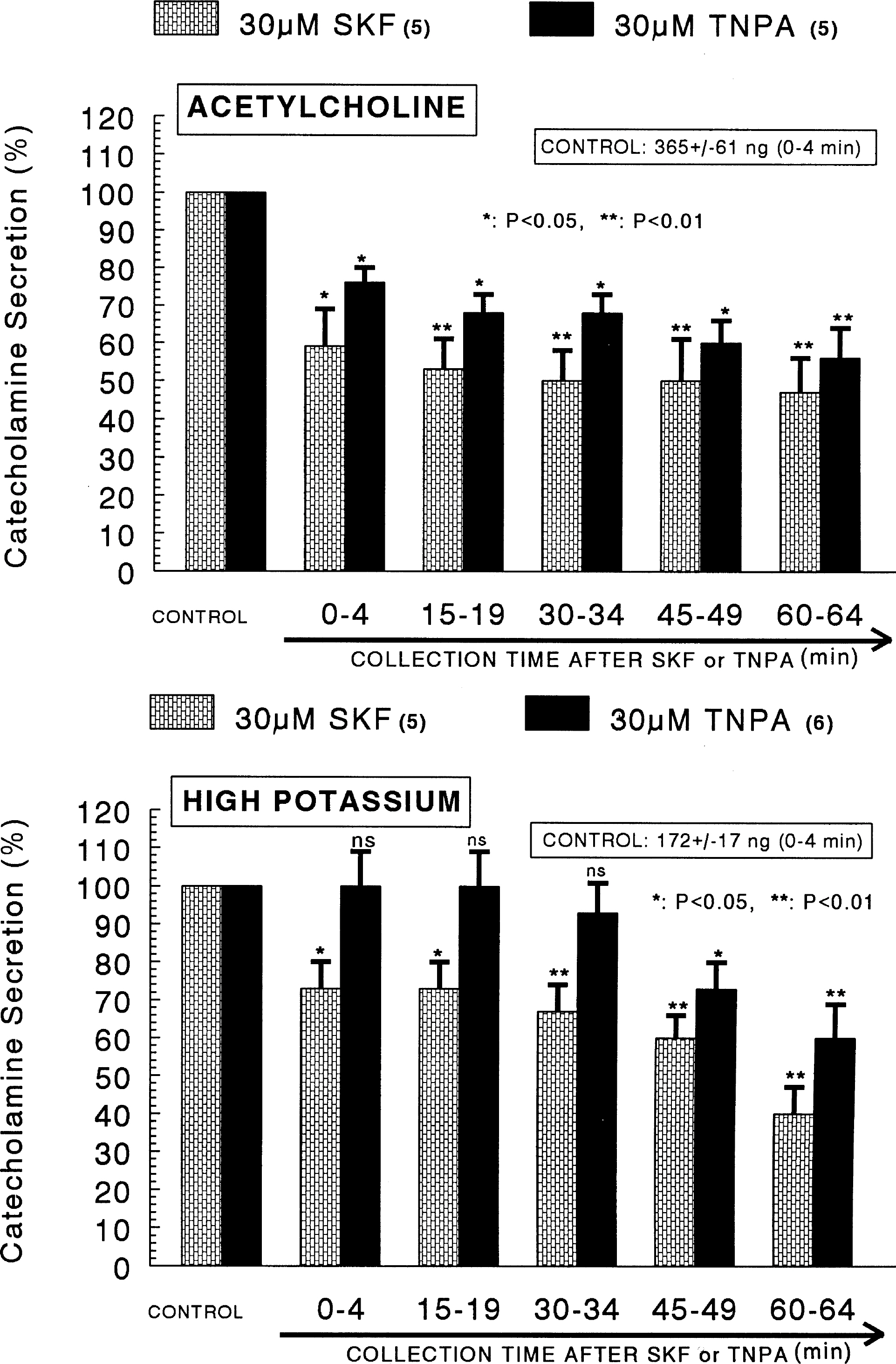
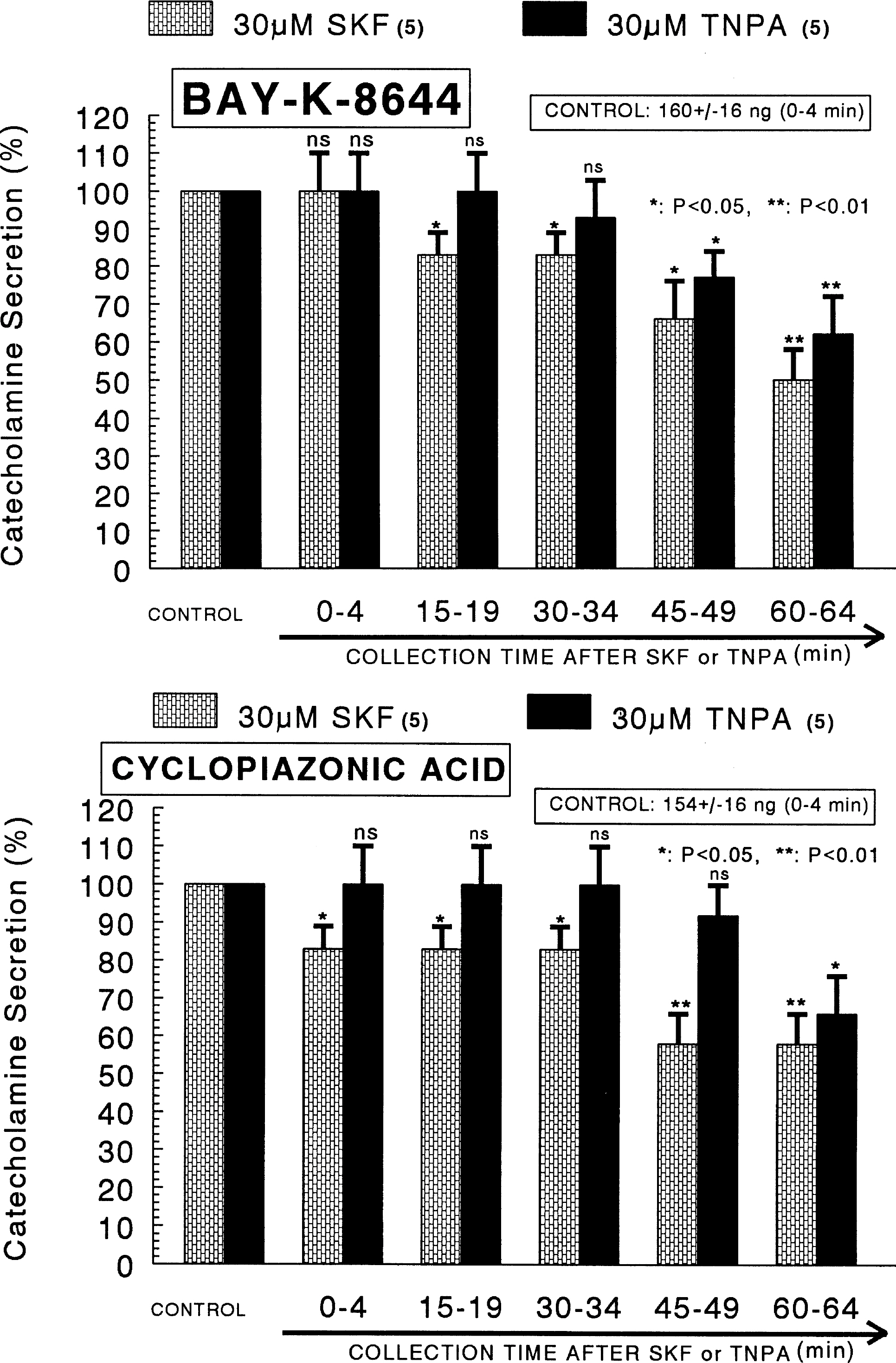
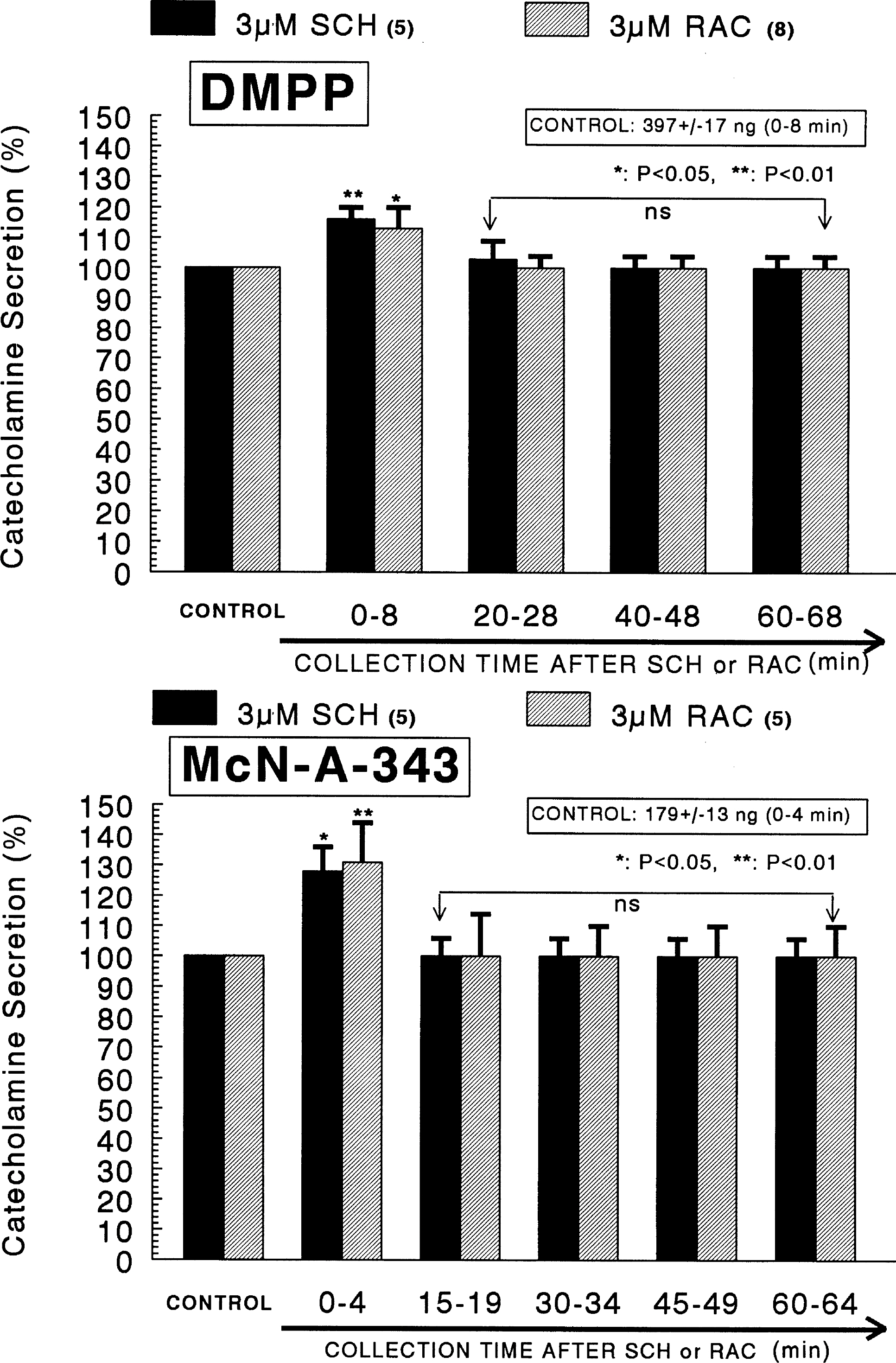
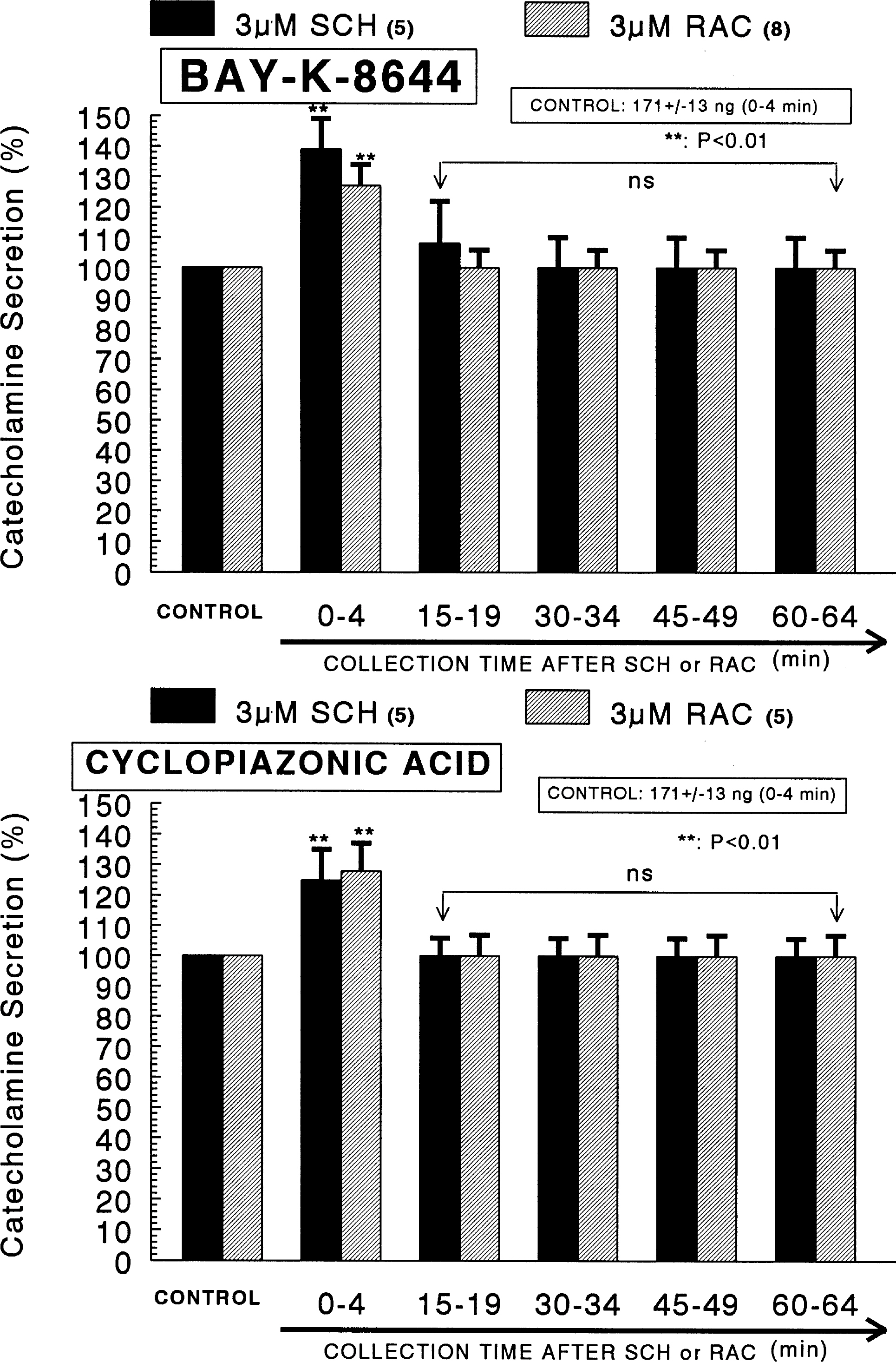
- The aim of the present study was designed to establish comparatively the inhibitory effects of D1-like and D2-like dopaminergic receptor agonists, SKF81297 and R(-)-TNPA on the release of catecholamines (CA) evoked by cholinergic stimulation and membrane depolarization from the isolated perfused model of the rat adrenal medulla. SKF81297 (30 micrometer) and R-(-)-TNPA (30 micrometer) perfused into an adrenal vein for 60 min, produced great inhibition in the CA secretory responses evoked by ACh (5.32x10(-3) M), DMPP (10(-4) M), McN-A-343 (10(-4) M), high K+ (5.6x10(-2) M), Bay-K-8644 (10 micrometer), and cyclopiazonic acid (10 micrometer), respectively. For the release of CA evoked by ACh, high K+, DMPP, McN-A-343, Bay-K-8644 and cyclopiazonic acid, the following rank order of inhibitory potency was obtained: SKF81297>R-(-)-TNPA. However, R(+)-SCH23390, a selectve D1-like dopaminergic receptor antagonist, and S(-)-raclopride, a selectve D2-like dopaminergic receptor antagonist, enhanced the CA secretory responses evoked by ACh, high K+, DMPP, McN-A-343, Bay-K-8644 and cyclopiazonic acid only for 0~4 min. The rank order for the enhancement of CA release evoked by high K+, McN-A-343 and cyclopiazonic acid was R(+)-SCH23390>S(-)-raclopride. Also, the rank order for ACh, DMPP and Bay-K-8644 was S(-)-raclopride > R(+)-SCH23390. Taken together, these results demonstrate that both SKF81297 and R-(-)-TNPA inhibit the CA release evoked by stimulation of cholinergic (both nicotinic and muscarinic) receptors and the membrane depolarization from the isolated perfused rat adrenal gland without affecting the basal release, respectively, but both R(+)-SCH23390 and S(-)-raclopride facilitate the CA release evoked by them. It seems likely that the inhibitory effects of SKF81297 and R-(-)-TNPA are mediated by the activation of D1-like and D2-like dopaminergic receptors located on the rat adrenomedullary chromaffin cells, respectively, whereas the facilitatory effects of R(+)-SCH23390 and S(-)-raclopride are mediated by the blockade of D1-like and D2-like dopaminergic receptors, respectively: this action is possibly associated with extra- and intracellular calcium mobilization. Based on these results, it is thought that the presence of dopaminergic D1 receptors may play an important role in regulation of the rat adrenomedullary CA secretion, in addition to well-known dopaminergic D2 receptors.
Keyword
MeSH Terms
-
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Adrenal Glands
Adrenal Medulla
Animals
Benzazepines
Calcium
Catecholamines
Chromaffin Cells
Dimethylphenylpiperazinium Iodide
Indoles
Membranes
Rats
Veins
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Benzazepines
Calcium
Catecholamines
Dimethylphenylpiperazinium Iodide
Indoles
Figure
Reference
-
Akaike A., Mine Y., Sasa M., Takaori S. Voltage and current clamp studies of muscarinic and nicotinic excitation of the rat adrenal chromaffin cells. J Pharmacol Expt Ther. 255:333–339. 1990.Albillos A., Abad F., Garcia AG. Cross-talk between M2 muscarinic and D1 dopamine receptors in the cat adrenal medulla. Biochem Biophys Res Commun. 183:1019–1024. 1992.Andersen PH., Jansen JA. Dopamine receptor agonists: selectivity and D1 receptor efficacy. Eur J Pharmacol. 188:335–347. 1990.Anton AH., Sayre DF. A study of the factors affecting the aluminum oxidetrihydroxy indole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 138:360–375. 1962.Artalejo CR., Ariano MA., Perlman RL., Fox AP. Activation of facilitation calcium channels in chromaffin cells by D1 dopamine receptors through a AMP/protein Kinase A-dependent mechanism. Nature. 348:239–242. 1990.Artalejo CR., Dahmer MK., Perlman RL., Fox AP. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol. 432:681–707. 1991.Artalejo CR., Garcia AG., Montiel C., Sanchez-Garcia P. A dopaminergic receptor modulates catecholamine release from the cat adrenal gland. J Physiol. 362:359–368. 1985.
ArticleBenoit-Marand M., Borrelli E., Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 21:9134–9141. 2001.Berridge MJ. Inositol trisphosphate and calcium signaling. Ann NY Acad Sci. 766:31–43. 1995.
ArticleBigornia L., Allen CN., Jan CR., Lyon RA., Titeler M., Schneider AS. D2 dopamine receptors modulate calcium channel currents and catecholamine secretion in bovine adrenal chromaffin cells. J Pharmacol Expt Ther. 252:586–592. 1990.Bigornia L., Suozzo M., Ryan KA., Napp D., Schneider AS. Dopamine receptors on adrenal chromaffin cells modulate calcium uptake and catecholamine release. J Neurochem. 51:999–1006. 1988.
ArticleChalliss RAJ., Jones JA., Owen PJ., Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 56:1083–1086. 1991.
ArticleCheek TR., O'Sullivan AJ., Moreton RB., Berridge MJ., Burgoyne RD. Spatial localization of the stimulus-induced rise in cyrosolic Ca2+ in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic patterns. FEBS Lett. 247:429–434. 1989.Chu E., Chu TC., Potter DE. Potential sites of action of TNPA: a dopamine-2 receptor agonist. Exp Eye Res. 69:611–616. 1999.
ArticleCollet AR., Story DF. Is catecholamine release from the rabbit adrenal gland subject to regulation through dopamine receptors or beta-adrenoceptors? Clin Exp Pharmacol Physiol. 9:436. 1982a.Collet AR., Story DF. Release of 3H-adrenaline from an isolated intact preparation of the rabbit adrenal gland: no evidence for release modulatory alpha-adrenoceptors. J Auton Pharmacol. 2:25–34. 1982b.Dahmer MK., Senogles SE. Atypical SCH23390 binding sites are present on bovine adrenal medullary membranes. Neurochem Res. 25:321–326. 2000.Dahmer MK., Senogles SE. Differential inhibition of secretagogue-stimulated sodium uptake in adrenal chromaffin cells by activation of D4 and D5 dopamine receptors. J Neurochem. 67:1960–1964. 1996.Dahmer MK., Senogles SE. Dopaminergic inhibition of catecholamine secretion from chromaffin cells: evidence that inhibition is mediated by D4 and D5 receptors. J Neurochem. 66:222–232. 1996.Damase-Michel C., Montastruc JL., Geelen G., Saint-Blanquat GD., Tran MA. Effect of quinpirole a specific dopamine DA2 receptor agonist on the sympathoadrenal system in dogs. J Pharmacol Expt Ther. 252:770–777. 1990.Damase-Michel C., Montastruc JL., Tran MA. Dopaminergic inhibition of catecholamine secretion from adrenal medulla is mediated by D2-like but not D1-like dopamine receptors. Clin Expt Pharmacol Physiol. 26(suppl):S67–S68. 1999.Damase-Michel C., Montastruc JL., Tran MA. Effects of dopa-minergic drugs on the sympathoadrenal system. Hypertens Res. 18(Suppl 1):S119–24. 1995.
ArticleFasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 15:77–83. 1994.Foucart S., Lacaille-Belanger P., Kimura T., Nadeau R., De Champlain J. Modulation of adrenal catecholamine release by D2 dopamine receptors in the anaesthetized dog. Clin Exp Pharmacol Physiol. 15:601–611. 1988.Garcia AG., Sala F., Reig JA., Viniegra S., Frias J., Fonteriz R., Gandia L. Ihydropyridine Bay-K-8644 activates chromaffin cell calcium channels. Nature. 309:69–71. 1984.Gessa L., Canu A., Del Zompo M., Burrai C., Serra G. Lack of acute antipsychotic effect of SCH23390, a selective dopamine D1 receptor antagonist. Lancet. 337:854–855. 1991.Goeger DE., Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 38:3995–4003. 1989.Gonzales MC., Artalejo AR., Montiel C., Hervas PP., Garcia AG. Characterization of a dopaminergic receptor that modulates adrenomedullary catecholamine release. J Neurochem. 47:382–388. 1986.Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 31:2992–2998. 1982.Huettl P., Gerhardt GA., Browning MD., Masserano JM. Effects of dopamine receptor agonists and antagonists on catecholamine release in bovine chromaffin cells. J Pharmacol Expt Ther. 257:567–574. 1991.Kujacic M., Carlsson A. Evidence for an increased catecholamine synthesis in rat adrenal glands following stimulation of peripheral dopamine receptors. J Neural Transm Gen Sect. 92:73–79. 1993.
ArticleKujacic M., Carlsson A. In vivo activity of tyrosine hydroxylase in rat adrenal glands following administration of quinpirole and dopamine. Eur J Pharmacol. 278:9–15. 1995.
ArticleKujacic M., Svensson K., Lofberg L., Carlsson A. Acute changes in dopamine levels in rat adrenal gland after administration of dopamine receptor agonists and antagonists. Eur J Pharmacol. 177:163–170. 1990.Kujacic M., Svensson K., Lofberg L., Carlsson A. Dopamine receptors, controlling dopamine levels in rat adrenal glands-comparison with central dopaminergic autoreceptors. J Neural Transm Gen Sect. 84:195–209. 1991.Lewis MM., Watts VJ., Lawler P., Nichols E., Mailman RB. Homologous desensitization of the D1A dopamine receptor: efficacy in causing desensitization dissociates from both receptor occupancy and functional potency. J Pharmacol Exp Ther. 286:345–353. 1998.Lim DY., Hwang DH. Studies on secretion of catecholamines evoked by DMPP and McN-A-343 in the rat adrenal gland. Korean J Pharmacol. 27:53–67. 1991.Lim DY., Kim CD., Ahn KW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 15:115–125. 1992.
ArticleLim DY., Kim KH., Choi CH., Yoo HJ., Choi DJ., Lee EH. Studies on secretion of catecholamines evoked by metolclopramide of the rat adrenal gland. Korean J Pharmacol. 25:31–42. 1989.Lim DY., Yoon JK., Moon B. Interrelationship between dopaminergic receptors and catecholamine secretion from the rat adrenal gland. Korean J Pharmacol. 30:87–100. 1994.Lyon RA., Titeler M., Bigornia L., Schneider AS. D2 dopamine receptors on bovine chromaffin cell membranes: identification and characterization by [3H] N-methylspiperone binding. J Neurochem. 48:631–635. 1987.Mannelli M., Lanzillotti R., Ianni L., Pupilli C., Serio M. Dopaminergic modulation of human adrenal medulla: indirect evidence for the involvement of DA-2 receptors located on chromaffin cells. J Auton Pharmacol 10 Suppl. 1:79–84. 1990.Mannelli M., Pupilli C., Fabbri G., Musante R., De Feo ML., Franchi F., Guisti G. Endogenous dopamine (DA) and DA2 receptors: a mechanism limiting excessive sympatheticadrenal discharge in humans. J Clin Endocrinol Metab. 66:626–631. 1988.
ArticleMercuro G., Gessa G., Rivano CA., Lai L., Cherchi A. Evidence for a dopaminergic control of sympathoadrenal catecholamine release. Am J Cardiol. 62(10 Pt 1):827–828. 1988.
ArticleMontastruc JL., Gaillard G., Rascol O., Tran MA., Montastruc P. Effect of apomorphine on adrenal medullary catecholamine levels. Fundam Clin Pharmacol. 3:665–670. 1989.
ArticleMontiel C., Artalejo AR., Bermejo PM., Sanchez-Garcia P. A dopaminergic receptor in adrenal medulla as a possible site of action for the droperidol-evoked hypertensive response. Anesthesiology. 65:474–479. 1986.
ArticleNagahama S., Chen YF., Lindheimer MD., Oparil S. Mechanism of the pressor action of LY171555, a specific dopamine D2 receptor agonist, in the conscious rat. J Pharmacol Exp Ther. 236:735–742. 1986.O'Boyle KM., Gaitanopoulos DE., Brenner M., Waddington JL. Agonist and antagonist properties of benzazepine and thienopyrine derivates at the D1 dopamine receptor. Neuropharmacol. 28:401–405. 1989.Quick M., Bergeron L., Mount H., Philte J. Dopamine D2 receptor binding in adrenal medulla: charadcterization using [3H] spiperone. Biochem Pharmacol. 36:3707–3713. 1987.Regunathan S., Missala K., Sourkes TL. Central regulation of adrenal tyrosine hydroxylase: effect of induction on catecholamine levels in the adrenal medulla and plasma. J Neurochem. 53:1706–1710. 1989.
ArticleSchoors DF., Vauquelin GP., De Vos H., Smets G., Velkeniers B., Vanhaelst L., Dupont AG. Identification of a D1 dopamine receptor, not linked to adenylate cyclase, on lactotroph cells. Br J Pharmacol. 103:1928–1934. 1991.Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 303:535–537. 1982.Seidler NW., Jona I., Vegh N., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasimc reticulum. J Biol Chem. 264:17816–17823. 1989.Starke K. Presynaptic autoreceptors in the third decade: focus on alpha2-adrenoceptors. J Neurochem. 78:685–693. 2001.Suzuki M., Muraki K., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 107:134–140. 1992.Takemura H., Hughes AR., Thastrup O., Putney JW Jr. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 264:12266–12271. 1989.Tallarida RJ., Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed.Speringer-Verlag;New York: p. p. 132. 1987.Villanueva M., Wightman RM. Facilitation of quantal release induced by a D1-like receptor on bovine chromaffin cells. Biochem. 46:3881–3887. 2007.Wada A., Takara H., Izumi F., Kobayashi H., Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience. 15:283–292. 1985.Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 313:463–480. 1981.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of SKF81297 on Catecholamine Release from the Perfused Rat Adrenal Medulla
- R-(-)-TNPA, a Dopaminergic D2 Receptor Agonist, Inhibits Catecholamine Release from the Rat Adrenal Medulla
- An Autoradiographic Study on the Rat Neostriatal Dopamine Receptor Changes after 6-hydroxydopamine Injection into the Medial Prefrontal Cortex
- Expression of Dopamine D1 and D2 Receptor mRNAs in the Fetal Rat Retina
- D-Amphetamine Causes Dual Actions on Catecholamine Release from the Rat Adrenal Medulla