Korean J Urol.
2015 Jan;56(1):31-40. 10.4111/kju.2015.56.1.31.
Expression of survivin in squamous cell carcinoma and transitional cell carcinoma of the urinary bladder: A comparative immunohistochemical study
- Affiliations
-
- 1Department of Pathology, Faculty of Medicine, Assiut University, Assiut, Egypt. rania_makboul@yahoo.com
- 2Department of Urology, Faculty of Medicine, Assiut University, Assiut, Egypt.
- 3Department of Urology and Urological Oncology, Hannover Medical School, Hannover, Germany.
- KMID: 2070266
- DOI: http://doi.org/10.4111/kju.2015.56.1.31
Abstract
- PURPOSE
To compare the expression of survivin and its association with clinicopathological criteria in major types of urinary bladder carcinoma, specifically, transitional cell carcinoma with and without squamous differentiation and squamous cell carcinoma.
MATERIALS AND METHODS
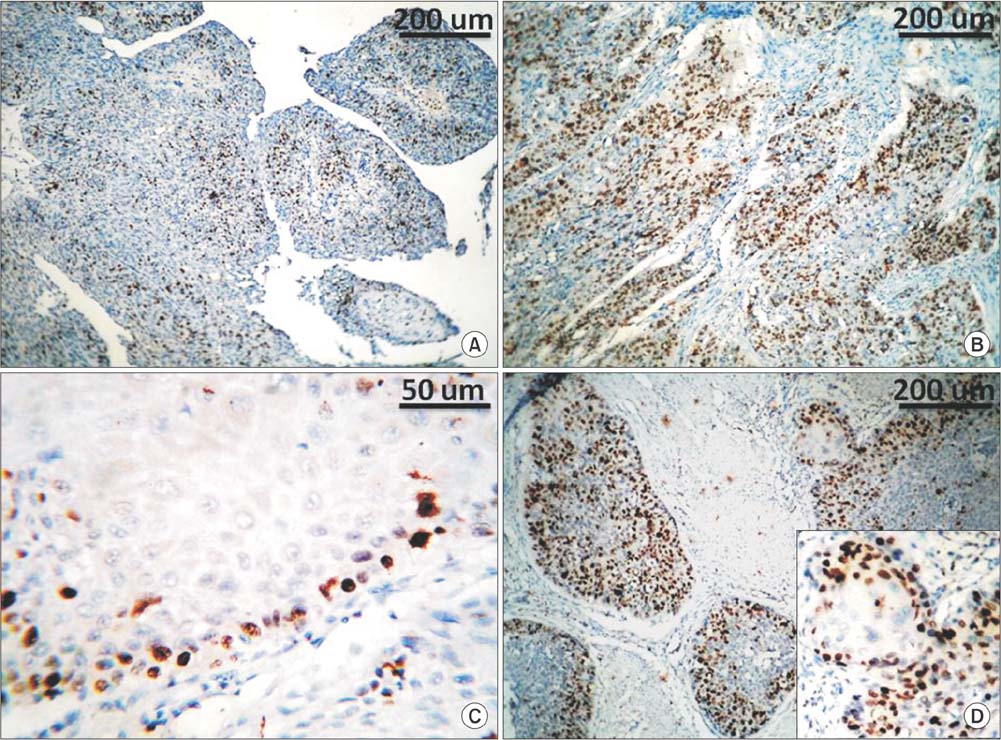
Immunohistochemical staining for survivin and Ki67 was performed on paraffin-embedded sections of 104 carcinomas: 52 transitional cell carcinoma, 20 transitional cell carcinoma with squamous differentiation, and 32 squamous cell carcinoma. Expression of survivin in >10% of tumor cells was described as altered survivin status. Ki67 staining in >20% of tumor cells was described as a high proliferation index.
RESULTS
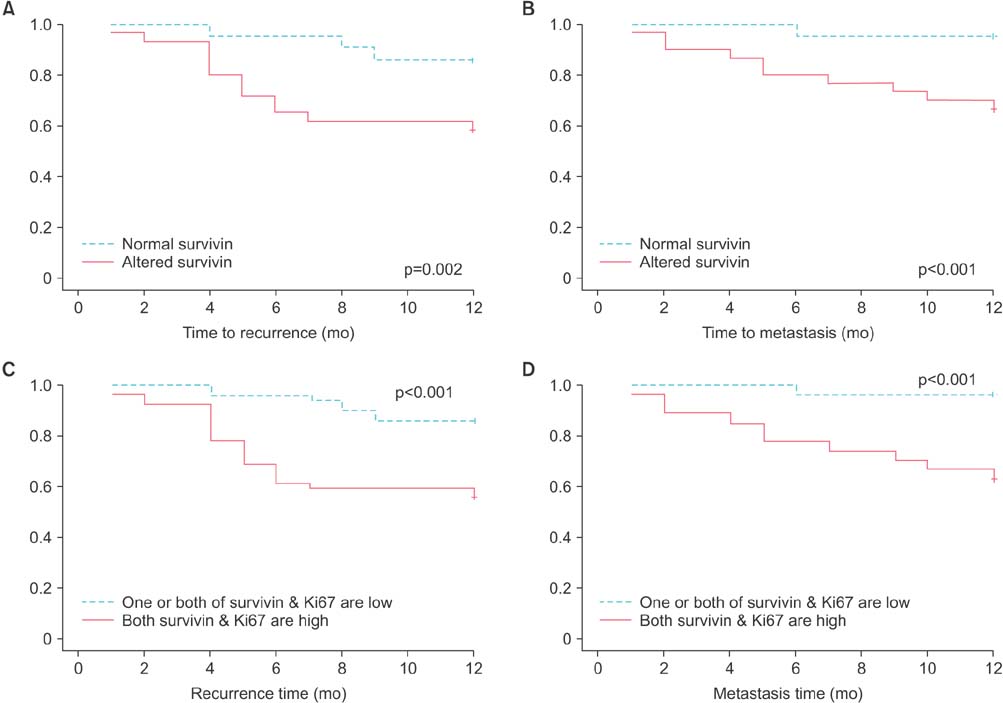
Altered survivin expression was detected in 60/104 specimens (58%) and was significantly more frequent in transitional cell carcinoma (78%) than in squamous cell carcinoma (38%) or transitional cell carcinoma with squamous differentiation (40%) (p<0.0001). In transitional cell carcinoma but not in squamous cell carcinoma, altered survivin status was associated with higher tumor grade, higher proliferation index, and recurrence. In the whole specimens, altered survivin expression was significantly associated with advanced stage (p<0.001), recurrence (p=0.005), distant metastasis (p<0.001), and death (p=0.001). In the multivariate analysis, altered survivin was an independent poor prognostic factor for recurrence.
CONCLUSIONS
Unlike in transitional cell carcinoma, alteration of survivin expression in squamous cell carcinoma occurs less frequently and is not associated with features of tumor aggression or patient outcome. These findings raise a question: are urinary bladder carcinoma patients with squamous cell carcinoma type suitable candidates for survivin vaccine? This is an important question to be answered before approving the vaccine in management.
MeSH Terms
-
Carcinoma, Squamous Cell/*genetics
Carcinoma, Transitional Cell/*genetics
Female
Humans
Inhibitor of Apoptosis Proteins/genetics/*metabolism
Ki-67 Antigen/metabolism
Male
Middle Aged
Multivariate Analysis
Neoplasm Grading
Neoplasm Metastasis
Neoplasm Recurrence, Local
Neoplasm Staging
Prognosis
Treatment Outcome
Tumor Markers, Biological
Urinary Bladder/pathology
Urinary Bladder Neoplasms/*genetics
Inhibitor of Apoptosis Proteins
Ki-67 Antigen
Tumor Markers, Biological
Figure
Reference
-
1. Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009; 27:289–293.2. Amr S, Dawson R, Saleh DA, Magder LS, Mikhail NN, St George DM, et al. Agricultural workers and urinary bladder cancer risk in Egypt. Arch Environ Occup Health. 2014; 69:3–10.3. Knowles MA. Molecular pathogenesis of bladder cancer. Int J Clin Oncol. 2008; 13:287–297.4. Rausch S, Lotan Y, Youssef RF. Squamous cell carcinogenesis and squamous cell carcinoma of the urinary bladder: a contemporary review with focus on nonbilharzial squamous cell carcinoma. Urol Oncol. 2014; 32:32.e11–32.e16.5. Karam JA, Lotan Y, Ashfaq R, Sagalowsky AI, Shariat SF. Survivin expression in patients with non-muscle-invasive urothelial cell carcinoma of the bladder. Urology. 2007; 70:482–486.6. Coumar MS, Tsai FY, Kanwar JR, Sarvagalla S, Cheung CH. Treat cancers by targeting survivin: just a dream or future reality? Cancer Treat Rev. 2013; 39:802–811.7. Srivastava AK, Singh PK, Srivastava K, Singh D, Dalela D, Rath SK, et al. Diagnostic role of survivin in urinary bladder cancer. Asian Pac J Cancer Prev. 2013; 14:81–85.8. Altieri DC. Targeting survivin in cancer. Cancer Lett. 2013; 332:225–228.9. Altieri DC. Survivin in apoptosis control and cell cycle regulation in cancer. Prog Cell Cycle Res. 2003; 5:447–452.10. Chen YB, Tu JJ, Kao J, Zhou XK, Chen YT. Survivin as a useful adjunct marker for the grading of papillary urothelial carcinoma. Arch Pathol Lab Med. 2008; 132:224–231.11. Charpin C, Habib MC, Piana L, Rampal M, Andrac L, Vacheret H, et al. Growth fraction (Ki67), ploidy balance and proliferation index in tumors of the urogenital tract and breast. Ann Pathol. 1989; 9:331–339.12. Eble JN, Sauter G, Epstein JL, Sesterhenn IA, editors. Pathology and genetics: tumours of the urinary system and male genital organs. Lyon: IARCPress, International Agency for Research on Cancer;2004. (World Health Organization classification of tumours; v. 6).13. Sobin LH, Gospodariwicz MK, Wittekind C. International Union against Cancer. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell;2009.14. Shariat SF, Bolenz C, Godoy G, Fradet Y, Ashfaq R, Karakiewicz PI, et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol. 2009; 182:78–84.15. Jeon HG, Jeong IG, Bae J, Lee JW, Won JK, Paik JH, et al. Expression of Ki-67 and COX-2 in patients with upper urinary tract urothelial carcinoma. Urology. 2010; 76:513.e7–513.e12.16. Jeon C, Kim M, Kwak C, Kim HH, Ku JH. Prognostic role of survivin in bladder cancer: a systematic review and meta-analysis. PLoS One. 2013; 8:e76719.17. Lv S, Turlova E, Zhao S, Kang H, Han M, Sun HS. Prognostic and clinicopathological significance of survivin expression in bladder cancer patients: a meta-analysis. Tumour Biol. 2014; 35:1565–1574.18. Swana HS, Grossman D, Anthony JN, Weiss RM, Altieri DC. Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N Engl J Med. 1999; 341:452–453.19. Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006; 244:164–171.20. Yin W, Chen N, Zhang Y, Zeng H, Chen X, He Y, et al. Survivin nuclear labeling index: a superior biomarker in superficial urothelial carcinoma of human urinary bladder. Mod Pathol. 2006; 19:1487–1497.21. Muscheck M, Abol-Enein H, Chew K, Moore D 2nd, Bhargava V, Ghoneim MA, et al. Comparison of genetic changes in schistosome-related transitional and squamous bladder cancers using comparative genomic hybridization. Carcinogenesis. 2000; 21:1721–1726.22. Abdulamir AS, Hafidh RR, Kadhim HS, Abubakar F. Tumor markers of bladder cancer: the schistosomal bladder tumors versus non-schistosomal bladder tumors. J Exp Clin Cancer Res. 2009; 28:27.23. Weiss C, von Romer F, Capalbo G, Ott OJ, Wittlinger M, Krause SF, et al. Survivin expression as a predictive marker for local control in patients with high-risk T1 bladder cancer treated with transurethral resection and radiochemotherapy. Int J Radiat Oncol Biol Phys. 2009; 74:1455–1460.24. Karam JA, Lotan Y, Karakiewicz PI, Ashfaq R, Sagalowsky AI, Roehrborn CG, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007; 8:128–136.25. Muschol-Steinmetz C, Friemel A, Kreis NN, Reinhard J, Yuan J, Louwen F. Function of survivin in trophoblastic cells of the placenta. PLoS One. 2013; 8:e73337.26. Kausch I, Bohle A. Molecular aspects of bladder cancer III. Prognostic markers of bladder cancer. Eur Urol. 2002; 41:15–29.27. Dudziec E, Goepel JR, Catto JW. Global epigenetic profiling in bladder cancer. Epigenomics. 2011; 3:35–45.28. Sjodahl G, Lauss M, Lovgren K, Chebil G, Gudjonsson S, Veerla S, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012; 18:3377–3386.29. Lo Muzio L, Farina A, Rubini C, Pezzetti F, Stabellini G, Laino G, et al. Survivin as prognostic factor in squamous cell carcinoma of the oral cavity. Cancer Lett. 2005; 225:27–33.30. Tanaka T, Kitamura H, Inoue R, Nishida S, Takahashi-Takaya A, Kawami S, et al. Potential survival benefit of anti-apoptosis protein: survivin-derived peptide vaccine with and without interferon alpha therapy for patients with advanced or recurrent urothelial cancer--results from phase I clinical trials. Clin Dev Immunol. 2013; 2013:262967.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Cyclooxygenase-2 and Survivin in Urinary Bladder Transitional Cell Carcinoma
- Correlation between Histopathologic Grade, Stage, and Degree of EGFR Expression in Transitional Cell Carcinoma of the Urinary Bladder
- A Case of Synchronous Squamous Cell Carcinoma of the Bladder and Transitional Cell Carcinoma of the Ureter
- Clinical Implications of the Expression of Survivin and p53 in Superficial Transitional Cell Carcinoma of the Bladder
- The Study of Ferritin Antigen and Carcinoembryonic Antigen in the Transitional Cell Carcinoma of Urinary Bladder by the Immunoperoxidase Technique