Yonsei Med J.
2014 Nov;55(6):1576-1583. 10.3349/ymj.2014.55.6.1576.
At Least One Cyclic Teriparatide Administration Can Be Helpful to Delay Initial Onset of a New Osteoporotic Vertebral Compression Fracture
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Orthopaedic Surgery, International St. Mary's Hospital, Catholic Kwandong University College of Medicine, Incheon, Korea. 144667@daum.net
- KMID: 2070205
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1576
Abstract
- PURPOSE
Teriparatide markedly increases bone formation and strength, while reducing the incidence of new-onset osteoporotic vertebral compression fractures (OVCFs). In some countries, expenses for teriparatide use are covered by medical insurance for up to 6 months; however, the national medical insurance of the authors' country does not cover these expenses. This retrospective cohort study compared the therapeutic effects of teriparatide on the initial onset of a new OVCF after treatment of osteoporosis and/or related OVCFs with regard to therapeutic durations of longer than 3 months (LT3M) or shorter than 3 months (ST3M).
MATERIALS AND METHODS
From May 2007 to February 2012, 404 patients who were prescribed and administered teriparatide and who could be followed-up for longer than 12 months were enrolled. They were divided into two groups depending on teriparatide duration: LT3M (n=132) and ST3M (n=272).
RESULTS
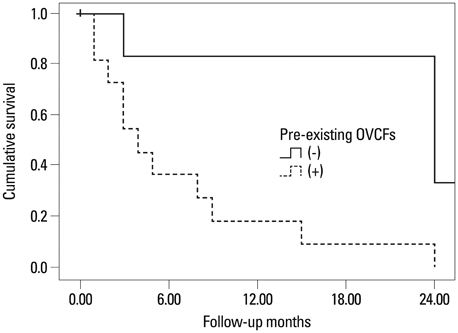
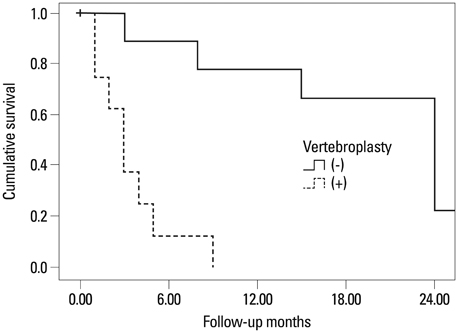
The group with the teriparatide duration of LT3M showed significantly less development of an initial OVCF within 1 year (p=0.004, chi-square). Duration of teriparatide use, body mass index, pre-teriparatide lowest spinal bone mineral density, and severity of osteoporosis significantly affected multiple regression analysis results (p<0.05). Survival analysis of first new-onset OVCFs demonstrated a significantly better survival rate for the LT3M group (log rank, p=0.005). Also, the ST3M group showed a higher odds ratio of 54.00 for development of an initial OVCF during follow-up than the LT3M group (Mantel-Haenzel common odds ratio, p=0.006).
CONCLUSION
At least one cyclic teriparatide administration is recommended to provide a protective effect against the initial onset of a new OVCF for up to one year after therapy.
MeSH Terms
-
Aged
Aged, 80 and over
Bone Density/drug effects
Bone Density Conservation Agents/*administration & dosage/pharmacology
Cohort Studies
Drug Administration Schedule
Female
Fractures, Compression/*drug therapy/etiology
Humans
Incidence
Male
Middle Aged
Osteoporosis/complications
Osteoporotic Fractures/*drug therapy/etiology
Retrospective Studies
Spinal Fractures/*drug therapy/etiology
Teriparatide/*administration & dosage/pharmacology
Time Factors
Treatment Outcome
Bone Density Conservation Agents
Teriparatide
Figure
Reference
-
1. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006; 367:2010–2018.
Article2. Hall SE, Criddle RA, Comito TL, Prince RL. A case-control study of quality of life and functional impairment in women with long-standing vertebral osteoporotic fracture. Osteoporos Int. 1999; 9:508–515.
Article3. Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, et al. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int. 2002; 13:527–536.
Article4. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999; 282:637–645.
Article5. Uihlein AV, Leder BZ. Anabolic therapies for osteoporosis. Endocrinol Metab Clin North Am. 2012; 41:507–525.
Article6. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996; 348:1535–1541.
Article7. Tseng YY, Su CH, Lui TN, Yeh YS, Yeh SH. Prospective comparison of the therapeutic effect of teriparatide with that of combined vertebroplasty with antiresorptive agents for the treatment of new-onset adjacent vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int. 2012; 23:1613–1622.
Article8. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001; 344:1434–1441.
Article9. Park JH, Kang KC, Shin DE, Koh YG, Son JS, Kim BH. Preventive effects of conservative treatment with short-term teriparatide on the progression of vertebral body collapse after osteoporotic vertebral compression fracture. Osteoporos Int. 2014; 25:613–618.
Article10. Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010; 95:1838–1845.
Article11. Kanis JA, McCloskey EV, Johansson H, Oden A, Ström O, Borgström F. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010; 21:Suppl 2. S407–S413.
Article12. Binkley N, Krueger D, Gangnon R, Genant HK, Drezner MK. Lateral vertebral assessment: a valuable technique to detect clinically significant vertebral fractures. Osteoporos Int. 2005; 16:1513–1518.
Article13. Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003; 18:1932–1941.
Article14. Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001; 16:1846–1853.
Article15. Nevitt MC, Chen P, Dore RK, Reginster JY, Kiel DP, Zanchetta JR, et al. Reduced risk of back pain following teriparatide treatment: a meta-analysis. Osteoporos Int. 2006; 17:273–280.
Article16. Nevitt MC, Chen P, Kiel DP, Reginster JY, Dore RK, Zanchetta JR, et al. Reduction in the risk of developing back pain persists at least 30 months after discontinuation of teriparatide treatment: a meta-analysis. Osteoporos Int. 2006; 17:1630–1637.
Article17. Ulivieri FM. Back pain treatment in post-menopausal osteoporosis with vertebral fractures. Aging Clin Exp Res. 2007; 19:3 Suppl. 21–23.18. Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007; 22:495–502.
Article19. Martin TJ, Sims NA, Ng KW. Regulatory pathways revealing new approaches to the development of anabolic drugs for osteoporosis. Osteoporos Int. 2008; 19:1125–1138.
Article20. Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med. 2005; 353:566–575.
Article21. Nshimyumukiza L, Durand A, Gagnon M, Douville X, Morin S, Lindsay C, et al. An economic evaluation: simulation of the cost-effectiveness and cost-utility of universal prevention strategies against osteoporosis-related fractures. J Bone Miner Res. 2013; 28:383–394.
Article22. Wasserfallen JB, Krieg MA, Greiner RA, Lamy O. Cost effectiveness and cost utility of risedronate for osteoporosis treatment and fracture prevention in women: a Swiss perspective. J Med Econ. 2008; 11:499–523.
Article23. McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005; 165:1762–1768.
Article24. Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002; 87:4528–4535.
Article25. Mudano AS, Bian J, Cope JU, Curtis JR, Gross TP, Allison JJ, et al. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: a population-based cohort study. Osteoporos Int. 2009; 20:819–826.
Article26. Boger A, Heini P, Windolf M, Schneider E. Adjacent vertebral failure after vertebroplasty: a biomechanical study of low-modulus PMMA cement. Eur Spine J. 2007; 16:2118–2125.
Article27. Buchbinder R, Kallmes DF. Vertebroplasty: when randomized placebo-controlled trial results clash with common belief. Spine J. 2010; 10:241–243.
Article28. Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009; 361:557–568.
Article29. Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009; 361:569–579.
Article30. Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006; 27:217–223.31. Voormolen MH, Lohle PN, Lampmann LE, van den Wildenberg W, Juttmann JR, Diekerhof CH, et al. Prospective clinical follow-up after percutaneous vertebroplasty in patients with painful osteoporotic vertebral compression fractures. J Vasc Interv Radiol. 2006; 17:1313–1320.
Article32. Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, et al. Early Postmenopausal Intervention Cohort (EPIC) study group. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. J Bone Miner Res. 1999; 14:1622–1627.
Article33. Moon ES, Kim HS, Park JO, Moon SH, Lee HM, Shin DE, et al. The incidence of new vertebral compression fractures in women after kyphoplasty and factors involved. Yonsei Med J. 2007; 48:645–652.
Article34. Zhang Z, Fan J, Ding Q, Wu M, Yin G. Risk factors for new osteoporotic vertebral compression fractures after vertebroplasty: a systematic review and meta-analysis. J Spinal Disord Tech. 2013; 26:E150–E157.35. Blick SK, Dhillon S, Keam SJ. Teriparatide: a review of its use in osteoporosis. Drugs. 2008; 68:2709–2737.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neglected Osteoporotic Vertebral Compression Fracture Treated with Postural Reduction and Minimally Invasive Transpedicular Fixation with Weekly Teriparatide
- The Factors between the Progression of the Compression Rate and Magnetic Resonance Imaging Findings in Osteoporotic Vertebral Fracture Patients Treated with Teriparatide
- Short-term Treatment Comparison of Teriparatide and Percutaneous Vertebroplasty in Patients with Acute Osteoporotic Vertebral Compression Fractures
- Effect of Weekly Teriparatide Administration Followed by Percutaneous Balloon Kyphoplasty on Post-Menopausal Osteoporotic Compression Fracture Treatment
- Can Three Months of Teriparatide Be One of Treatment Options for Osteoporotic Vertebral Compression Fracture Patients?