Yonsei Med J.
2014 Nov;55(6):1507-1515. 10.3349/ymj.2014.55.6.1507.
Lipoprotein-Associated Phospholipase A2 Is Related to Plaque Stability and Is a Potential Biomarker for Acute Coronary Syndrome
- Affiliations
-
- 1Cardiology Division, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. cardiobk@yuhs.ac
- 2Cardiology Division, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Cardiology Division, Department of Internal Medicine, Chungju Medical Center, Chungju, Korea.
- 4Cardiology Division, Department of Internal Medicine, NHIC Ilsan Hospital, Goyang, Korea.
- 5Department of Biochemistry, CHA University College of Medicine, Seongnam, Korea.
- KMID: 2070197
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1507
Abstract
- PURPOSE
Plasma lipoprotein-associated phospholipase A2 (Lp-PLA2) binds to low-density lipoprotein. The levels of Lp-PLA2 reflect the plaque burden, and are upregulated in acute coronary syndrome (ACS). We investigated the diagnostic value of Lp-PLA2 levels and found that it might be a potential biomarker for ACS.
MATERIALS AND METHODS
We classified 226 study participants into three groups: patients without significant stenosis (control group), patients with significant stenosis with stable angina (SA group), and patients with ACS (ACS group).
RESULTS
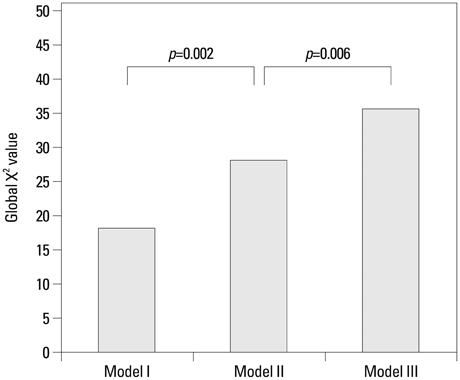
Lp-PLA2 and high-sensitivity C-reactive protein (hs-CRP) levels were significantly greater in the ACS group than in the SA group (p=0.044 and p=0.029, respectively). Multivariate logistic regression analysis revealed that Lp-PLA2 levels are significantly associated with ACS (odds ratio=1.047, p=0.013). The addition of Lp-PLA2 to the ACS model significantly increased the global chi2 value over traditional risk factors (28.14 to 35.602, p=0.006). The area under the receiver operating characteristic curve for Lp-PLA2 was 0.624 (p=0.004). The addition of Lp-PLA2 level to serum hs-CRP concentration yielded an integrated discrimination improvement of 0.0368 (p=0.0093, standard error: 0.0142) and improved the ability to diagnose ACS.
CONCLUSION
Lp-PLA2 levels are related to plaque stability and might be a diagnostic biomarker for ACS.
MeSH Terms
-
1-Alkyl-2-acetylglycerophosphocholine Esterase/*blood
Acute Coronary Syndrome/*blood/physiopathology
Aged
Aged, 80 and over
Angina Pectoris
Biological Markers/blood
C-Reactive Protein/*metabolism
Coronary Angiography
Female
Humans
Lipoproteins, LDL/*blood
Logistic Models
Male
Middle Aged
Multivariate Analysis
Plaque, Atherosclerotic/blood
ROC Curve
Risk Factors
1-Alkyl-2-acetylglycerophosphocholine Esterase
Biological Markers
Lipoproteins, LDL
C-Reactive Protein
Figure
Reference
-
1. WRITING GROUP MEMBERS. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010; 121:e46–e215.2. Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008; 201:1–7.
Article3. Epps KC, Wilensky RL. Lp-PLA2- a novel risk factor for high-risk coronary and carotid artery disease. J Intern Med. 2011; 269:94–106.
Article4. Caslake MJ, Packard CJ, Robertson M, Cooney J, Nelson JJ, Ford I, et al. Lipoprotein-associated phospholipase A(2), inflammatory biomarkers, and risk of cardiovascular disease in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). Atherosclerosis. 2010; 210:28–34.
Article5. Packard CJ. Lipoprotein-associated phospholipase A2 as a biomarker of coronary heart disease and a therapeutic target. Curr Opin Cardiol. 2009; 24:358–363.
Article6. Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004; 109:837–842.
Article7. Koenig W, Khuseyinova N, Löwel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004; 110:1903–1908.
Article8. Serruys PW, García-García HM, Buszman P, Erne P, Verheye S, Aschermann M, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008; 118:1172–1182.
Article9. Liu YS, Hu XB, Li HZ, Jiang WD, Wang X, Lin H, et al. Association of lipoprotein-associated phospholipase A2 with characteristics of vulnerable coronary atherosclerotic plaques. Yonsei Med J. 2011; 52:914–922.
Article10. Gotsman I, Stabholz A, Planer D, Pugatsch T, Lapidus L, Novikov Y, et al. Serum cytokine tumor necrosis factor-alpha and interleukin-6 associated with the severity of coronary artery disease: indicators of an active inflammatory burden? Isr Med Assoc J. 2008; 10:494–498.11. Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006; 26:2523–2529.
Article12. Wilensky RL, Macphee CH. Lipoprotein-associated phospholipase A(2) and atherosclerosis. Curr Opin Lipidol. 2009; 20:415–420.
Article13. Braunwald E. Unstable angina. A classification. Circulation. 1989; 80:410–414.
Article14. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007; 116:e148–e304.15. Hamm CW, Braunwald E. A classification of unstable angina revisited. Circulation. 2000; 102:118–122.
Article16. Dada N, Kim NW, Wolfert RL. Lp-PLA2: an emerging biomarker of coronary heart disease. Expert Rev Mol Diagn. 2002; 2:17–22.
Article17. Caslake MJ, Packard CJ. Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol. 2003; 14:347–352.
Article18. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008; 27:157–172.
Article19. Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009; 150:795–802.
Article20. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32:2999–3054.21. Mannheim D, Herrmann J, Versari D, Gössl M, Meyer FB, McConnell JP, et al. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke. 2008; 39:1448–1455.
Article22. Stafforini DM, Tjoelker LW, McCormick SP, Vaitkus D, McIntyre TM, Gray PW, et al. Molecular basis of the interaction between plasma platelet-activating factor acetylhydrolase and low density lipoprotein. J Biol Chem. 1999; 274:7018–7024.
Article23. Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005; 25:923–931.24. Lp-PLA(2) Studies Collaboration. Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010; 375:1536–1544.
Article25. Tellis CC, Tselepis AD. The role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim Biophys Acta. 2009; 1791:327–338.
Article26. Häkkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999; 19:2909–2917.27. Tew DG, Southan C, Rice SQ, Lawrence MP, Li H, Boyd HF, et al. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins. Arterioscler Thromb Vasc Biol. 1996; 16:591–599.
Article28. Liu CF, Qin L, Ren JY, Chen H, Wang WM, Liu J, et al. Elevated plasma lipoprotein-associated phospholipase A2 activity is associated with plaque rupture in patients with coronary artery disease. Chin Med J (Engl). 2011; 124:2469–2473.29. Davidson MH, Corson MA, Alberts MJ, Anderson JL, Gorelick PB, Jones PH, et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol. 2008; 101:51F–57F.
Article30. Jung CH, Lee WY, Kim BY, Park SE, Rhee EJ, Park CY, et al. The risk of metabolic syndrome according to the white blood cell count in apparently healthy Korean adults. Yonsei Med J. 2013; 54:615–620.
Article31. Seo HS. The role and clinical significance of high-sensitivity C-reactive protein in cardiovascular disease. Korean Circ J. 2012; 42:151–153.
Article32. Owens AP 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012; 122:558–568.
Article33. Ryu SK, Mallat Z, Benessiano J, Tedgui A, Olsson AG, Bao W, et al. Phospholipase A2 enzymes, high-dose atorvastatin, and prediction of ischemic events after acute coronary syndromes. Circulation. 2012; 125:757–766.
Article34. Wilensky RL, Shi Y, Mohler ER 3rd, Hamamdzic D, Burgert ME, Li J, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008; 14:1059–1066.
Article35. Mohler ER 3rd, Ballantyne CM, Davidson MH, Hanefeld M, Ruilope LM, Johnson JL, et al. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008; 51:1632–1641.
Article36. Daida H, Iwase T, Yagi S, Ando H, Nakajima H. Effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity in Japanese dyslipidemic patients, with exploratory analysis of a PLA2G7 gene polymorphism of Val279Phe. Circ J. 2013; 77:1518–1525.
Article37. White H, Held C, Stewart R, Watson D, Harrington R, Budaj A, et al. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with coronary heart disease. Am Heart J. 2010; 160:655–661.
Article38. Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J. 2012; 33:2899–2909.
Article39. O'Donoghue ML, Braunwald E, White HD, Serruys P, Steg PG, Hochman J, et al. Study design and rationale for the Stabilization of pLaques usIng Darapladib-Thrombolysis in Myocardial Infarction (SOLID-TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J. 2011; 162:613–619.40. Drakopoulou M, Toutouzas K, Stefanadi E, Tsiamis E, Tousoulis D, Stefanadis C. Association of inflammatory markers with angiographic severity and extent of coronary artery disease. Atherosclerosis. 2009; 206:335–339.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Production of Phospholipase A2 in Different Types of Cultured Human Intervertebral Disc Cells
- Early Differential Changes in Coronary Plaque Composition According to Plaque Stability Following Statin Initiation in Acute Coronary Syndrome: Classification and Analysis by Intravascular Ultrasound-Virtual Histology
- Plaque Morphology in Acute Coronary Syndrome: An Intravascular Ultrasound Study
- Acute coronary syndrome and vulnerable plaque
- The Potential Role of Cardiac CT in Patients with Acute Coronary Syndrome