Yonsei Med J.
2014 Nov;55(6):1489-1497. 10.3349/ymj.2014.55.6.1489.
Concurrent Chemoradiotherapy Shows Long-Term Survival after Conversion from Locally Advanced to Resectable Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Liver Cancer Special Clinic, Yonsei University College of Medicine, Seoul, Korea. jsseong@yuhs.ac
- 2Department of Internal Medicine, Yonsei Liver Cancer Special Clinic, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Yonsei Liver Cancer Special Clinic, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Pathology, Yonsei Liver Cancer Special Clinic, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2070195
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1489
Abstract
- PURPOSE
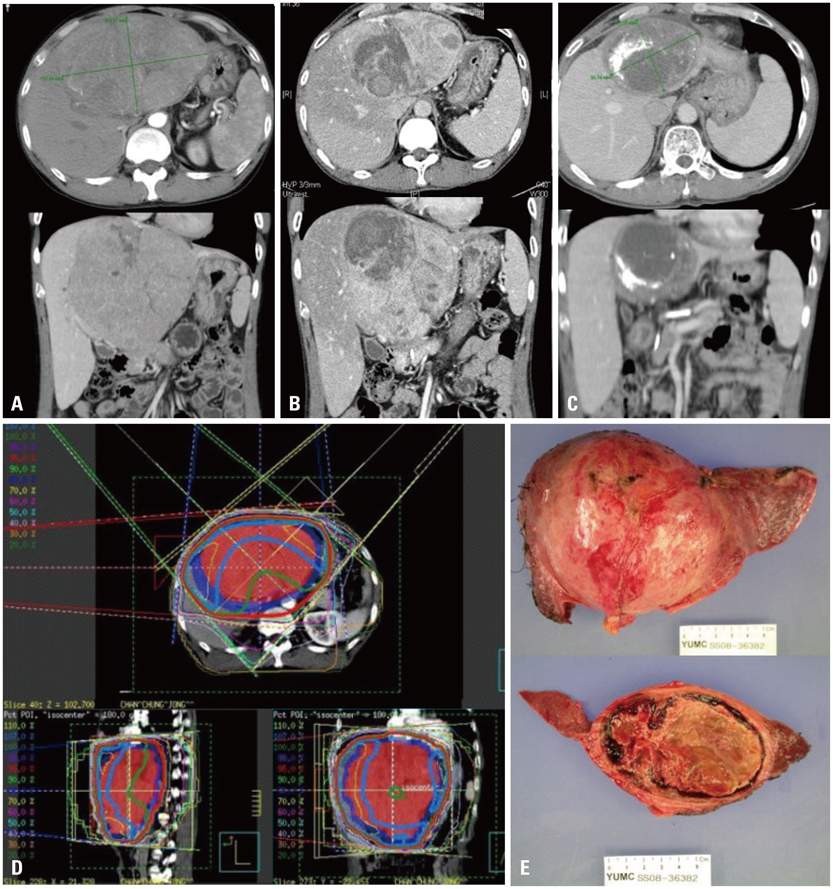
For locally unresectable hepatocellular carcinoma (HCC) patients, concurrent chemoradiotherapy (CCRT) has been applied as a loco-regional treatment. After shrinkage of tumors in selected patients, surgical resection is performed. The aim of this study was to evaluate prognostic factors and long-term survivors in such patients.
MATERIALS AND METHODS
From January 2000 to January 2009, 264 patients with HCC were treated with CCRT (45 Gy with fractional dose of 1.8 Gy), and intra-arterial chemotherapy was administered during radiotherapy. Eighteen of these patients (6.8%) underwent hepatic resection after showing a response to CCRT. Cases were considered resectable when tumor-free margins and sufficient remnant volumes were obtained without extrahepatic metastasis. Prior to operation, there were six patients with complete remission, 11 with partial remission, and six with stable disease according to modified Response Evaluation Criteria in Solid Tumors.
RESULTS
In pathologic review, four patients (22.2%) showed total necrosis and seven patients (38.9%) showed 70-99% necrosis. A high level of necrosis (> or =80%) was correlated with low risk for extrahepatic metastasis and long-term survival. In univariate analyses, vessel invasion and capsular infiltration were significantly correlated with disease free survival (DFS) (p=0.017 and 0.013, respectively), and vessel invasion was significantly correlated with overall survival (OS) (p=0.013). In multivariate analyses, capsule infiltration was a significant factor for DFS (p=0.016) and vessel invasion was significant for OS (p=0.015).
CONCLUSION
CCRT showed favorable responses and locally advanced HCC converted into resectable tumor after CCRT in selected patients. Long-term survivors showed the pathological features of near total necrosis, as well as negative capsule and vessel invasion.
Keyword
MeSH Terms
-
Adult
Aged
Antimetabolites, Antineoplastic/administration & dosage
Antineoplastic Agents/administration & dosage
Carcinoma, Hepatocellular/mortality/pathology/*therapy
Chemoradiotherapy/*methods
Cisplatin/administration & dosage
Disease-Free Survival
Female
Fluorouracil/administration & dosage
Humans
Liver Neoplasms/mortality/pathology/*therapy
Male
Middle Aged
Prognosis
Radiotherapy, Conformal
Remission Induction
Republic of Korea/epidemiology
*Salvage Therapy
Survival Rate
Treatment Outcome
Tumor Burden
Antimetabolites, Antineoplastic
Antineoplastic Agents
Cisplatin
Fluorouracil
Figure
Cited by 1 articles
-
Re-Irradiation of Hepatocellular Carcinoma: Clinical Applicability of Deformable Image Registration
Dong Soo Lee, Joong-Yeol Woo, Jun Won Kim, Jinsil Seong
Yonsei Med J. 2016;57(1):41-49. doi: 10.3349/ymj.2016.57.1.41.
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
Article2. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article3. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
Article4. Lee IJ, Seong J. Radiotherapeutic strategies in the management of hepatocellular carcinoma. Oncology. 2011; 81:Suppl 1. 123–133.
Article5. Lee IJ, Seong J. Radiosensitizers in hepatocellular carcinoma. Semin Radiat Oncol. 2011; 21:303–311.
Article6. Tang ZY, Yu YQ, Zhou XD, Ma ZC, Lu JZ, Liu KD, et al. Cytoreduction and sequential resection: a hope for unresectable primary liver cancer. J Surg Oncol. 1991; 47:27–31.
Article7. Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997; 226:688–701.
Article8. Huang J, He X, Lin X, Zhang C, Li J. Effect of preoperative transcatheter arterial chemoembolization on tumor cell activity in hepatocellular carcinoma. Chin Med J (Engl). 2000; 113:446–448.9. Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998; 15:674–678.
Article10. Han KH, Seong J, Kim JK, Ahn SH, Lee do Y, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008; 113:995–1003.
Article11. Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993; 9:298–304.
Article12. Choi GH, Han DH, Kim DH, Choi SB, Kang CM, Kim KS, et al. Outcome after curative resection for a huge (>or=10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg. 2009; 198:693–701.
Article13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.
Article14. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999; 19:329–338.
Article15. Lawrence TS, Tesser RJ, ten Haken RK. An application of dose volume histograms to the treatment of intrahepatic malignancies with radiation therapy. Int J Radiat Oncol Biol Phys. 1990; 19:1041–1047.
Article16. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991; 21:109–122.
Article17. Seong J, Keum KC, Han KH, Lee DY, Lee JT, Chon CY, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999; 43:393–397.
Article18. Kim YI, Park HC, Lim do H, Park HJ, Kang SW, Park SY, et al. Changes of the liver volume and the Child-Pugh score after high dose hypofractionated radiotherapy in patients with small hepatocellular carcinoma. Radiat Oncol J. 2012; 30:189–196.
Article19. Han KH, Lee JT, Seong J. Treatment of non-resectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2002; 17:Suppl 3. S424–S427.
Article20. Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005; 23:8739–8747.
Article21. Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000; 18:2210–2218.
Article22. Yoon HI, Koom WS, Lee IJ, Jeong K, Chung Y, Kim JK, et al. The significance of ICG-R15 in predicting hepatic toxicity in patients receiving radiotherapy for hepatocellular carcinoma. Liver Int. 2012; 32:1165–1171.
Article23. Lee IJ, Seong J, Shim SJ, Han KH. Radiotherapeutic parameters predictive of liver complications induced by liver tumor radiotherapy. Int J Radiat Oncol Biol Phys. 2009; 73:154–158.
Article24. Lee IJ, Seong J, Shim SJ, Han KH, Chon CY. [Reappraisal of risk factors predicting liver complications from radiotherapy for hepatocellular carcinoma]. Korean J Hepatol. 2006; 12:420–428.25. Wellwood JM, Cady B, Oberfield RA. Treatment of primary liver cancer: response to regional chemotherapy. Clin Oncol. 1979; 5:25–31.26. Atiq OT, Kemeny N, Niedzwiecki D, Botet J. Treatment of unresectable primary liver cancer with intrahepatic fluorodeoxyuridine and mitomycin C through an implantable pump. Cancer. 1992; 69:920–924.
Article27. Sandroussi C, Dawson LA, Lee M, Guindi M, Fischer S, Ghanekar A, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010; 23:299–306.
Article28. Wigg A, Hon K, Mosel L, Sladden N, Palumbo K. Down-staging of hepatocellular carcinoma via external-beam radiotherapy with subsequent liver transplantation: a case report. Liver Transpl. 2013; 19:1119–1124.
Article29. O'Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012; 18:949–954.30. Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005; 140:450–457.
Article31. Nagano Y, Tanaka K, Togo S, Matsuo K, Kunisaki C, Sugita M, et al. Efficacy of hepatic resection for hepatocellular carcinomas larger than 10 cm. World J Surg. 2005; 29:66–71.
Article32. Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007; 14:2817–2823.
Article33. Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009; 50:601–612.
Article34. Choi SB, Kim KS, Park YN, Choi JS, Lee WJ, Seong J, et al. The efficacy of hepatic resection after neoadjuvant transarterial chemoembolization (TACE) and radiation therapy in hepatocellular carcinoma greater than 5 cm in size. J Korean Med Sci. 2009; 24:242–247.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- A Case of Concurrent Chemoradiation Therapy for Locally Advanced Hepatocellular Carcinoma with Portal Vein Thrombosis
- Long-term survival after CCRT and HAIC followed by ALPPS for hepatocellular carcinoma with portal vein invasion: a case report
- Multidisciplinary Management of the Locally Advanced Unresectable Non-Small Cell Lung Cancer
- Role of Neoadjuvant Therapy for Borderline Resectable or Locally Advanced Pancreatic Cancer