Korean J Radiol.
2015 Feb;16(1):80-89. 10.3348/kjr.2015.16.1.80.
The Role of Three-Dimensional Multidetector CT Gastrography in the Preoperative Imaging of Stomach Cancer: Emphasis on Detection and Localization of the Tumor
- Affiliations
-
- 1Department of Radiology, Chonnam National University Medical School, Gwangju 501-757, Korea. kjradsss@dreamwiz.com
- 2Center for Aging and Geriatrics, Chonnam National University Medical School, Gwangju 501-757, Korea.
- 3Department of Radiology, Chonnam National University Hospital, Gwangju 501-757, Korea.
- 4Department of Surgery, Chonnam National University Medical School, Gwangju 501-757, Korea.
- KMID: 2069986
- DOI: http://doi.org/10.3348/kjr.2015.16.1.80
Abstract
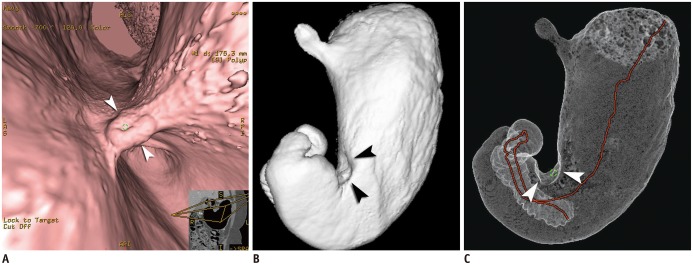
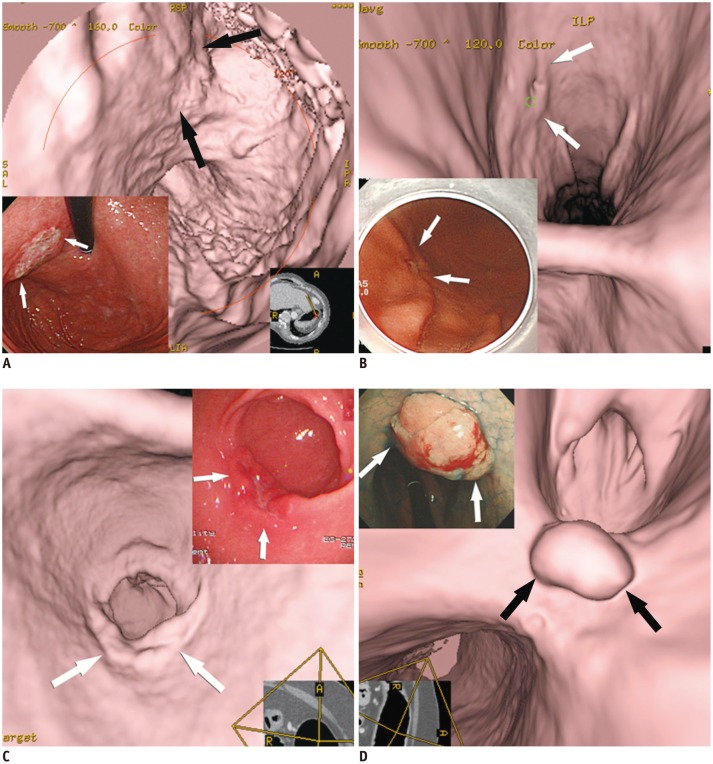
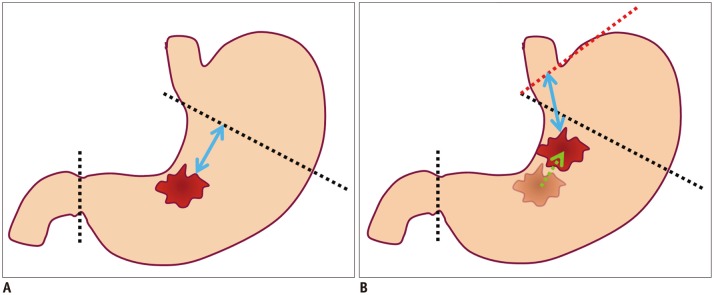
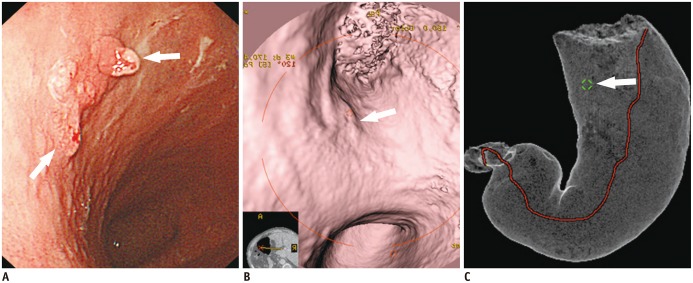
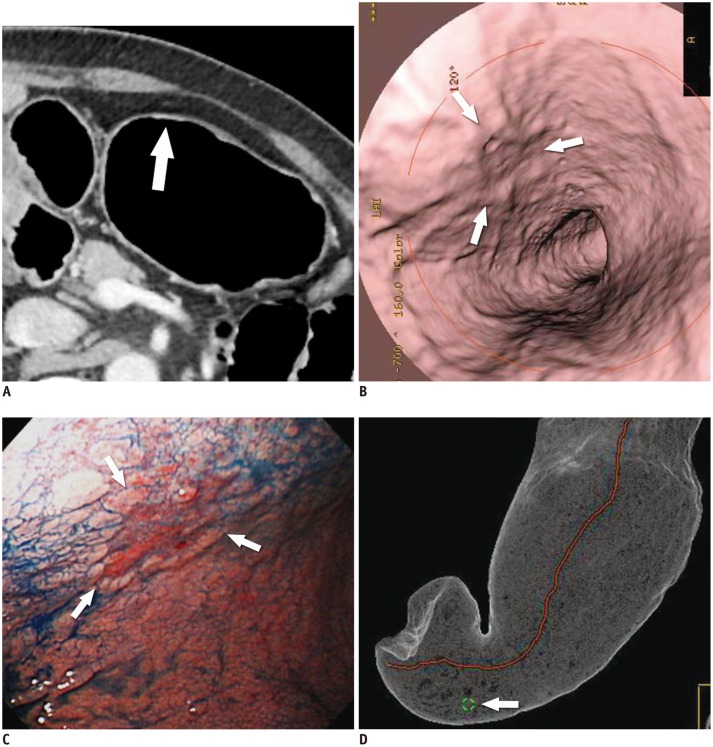
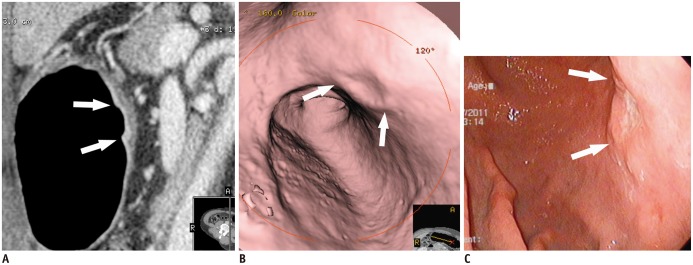
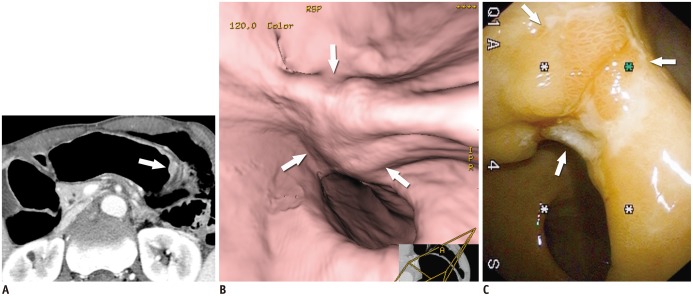
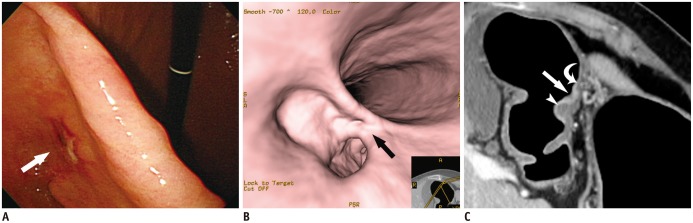
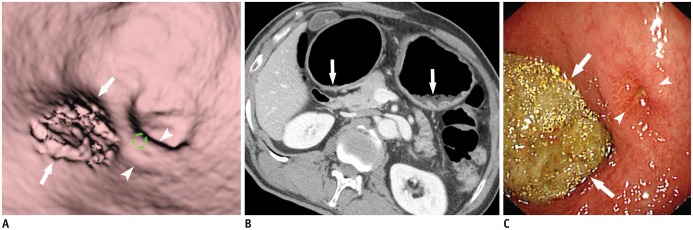
- Multidetector CT (MDCT) gastrography has been regarded as a promising technique for the preoperative imaging of gastric cancer. It has the ability to produce various three-dimensional (3D) images. Because 3D reconstruction images are more effective and intuitive for recognizing abnormal changes in the gastric folds and subtle mucosal nodularity than two-dimensional images, 3D MDCT gastrography can enhance the detection rate of early gastric cancer, which, in turn, contributes to the improvement of the accuracy of preoperative tumor (T) staging. In addition, shaded surface display and tissue transition projection images provide a global view of the stomach, with the exact location of gastric cancer, which may replace the need for barium studies. In this article, we discuss technical factors in producing high-quality MDCT gastrographic images and present cases demonstrating the usefulness of MDCT gastrography for the detection and T staging of gastric cancer while emphasizing the significance of preoperative localization of gastric cancer in terms of surgical margin.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effect of Preoperative Tumor Under-Staging on the Long-term Survival of Patients Undergoing Radical Gastrectomy for Gastric Cancer
Mi Lin, Qi-Yue Chen, Chao-Hui Zheng, Ping Li, Jian-Wei Xie, Jia-Bin Wang, Jian-Xian Lin, Chang-Ming Huang
Cancer Res Treat. 2021;53(4):1123-1133. doi: 10.4143/crt.2020.651.
Reference
-
1. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010; 19:1893–1907. PMID: 20647400.
Article2. Chung HW, Noh SH, Lim JB. Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol. 2010; 16:256–263. PMID: 20066747.
Article3. Kim JW, Shin SS, Heo SH, Choi YD, Lim HS, Park YK, et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur Radiol. 2012; 22:654–662. PMID: 21965037.
Article4. Lee IJ, Lee JM, Kim SH, Shin CI, Lee JY, Kim SH, et al. Diagnostic performance of 64-channel multidetector CT in the evaluation of gastric cancer: differentiation of mucosal cancer (T1a) from submucosal involvement (T1b and T2). Radiology. 2010; 255:805–814. PMID: 20501718.
Article5. Shen Y, Kang HK, Jeong YY, Heo SH, Han SM, Chen K, et al. Evaluation of early gastric cancer at multidetector CT with multiplanar reformation and virtual endoscopy. Radiographics. 2011; 31:189–199. PMID: 21257941.
Article6. Park HS, Lee JM, Kim SH, Lee JY, Yang HK, Han JK, et al. Three-dimensional MDCT for preoperative local staging of gastric cancer using gas and water distention methods: a retrospective cohort study. AJR Am J Roentgenol. 2010; 195:1316–1323. PMID: 21098189.
Article7. Kim SH, Han JK, Lee KH, Chung JW, Yang HK, Choi BI. Computed tomography gastrography with volume-rendering technique: correlation with double-contrast barium study and conventional gastroscopy. J Comput Assist Tomogr. 2003; 27:140–149. PMID: 12703002.
Article8. Kim HJ, Kim AY, Lee JH, Yook JH, Yu ES, Ha HK. Positioning during CT gastrography in patients with gastric cancer: the effect on gastric distension and lesion conspicuity. Korean J Radiol. 2009; 10:252–259. PMID: 19412513.
Article9. Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL, Chen J, et al. Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol. 2009; 100:205–214. PMID: 19530124.
Article10. Kim JH, Eun HW, Choi JH, Hong SS, Kang W, Auh YH. Diagnostic performance of virtual gastroscopy using MDCT in early gastric cancer compared with 2D axial CT: focusing on interobserver variation. AJR Am J Roentgenol. 2007; 189:299–305. PMID: 17646454.
Article11. Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT--correlation with surgical and histopathologic results. Radiology. 2007; 242:472–482. PMID: 17255419.
Article12. Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim PN, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005; 236:879–885. PMID: 16020558.
Article13. Kim SH, Lee JM, Han JK, Lee JY, Yang HK, Lee HJ, et al. Effect of adjusted positioning on gastric distention and fluid distribution during CT gastrography. AJR Am J Roentgenol. 2005; 185:1180–1184. PMID: 16247129.
Article14. Yu JS, Choi SH, Choi WH, Chung JJ, Kim JH, Kim KW. Value of nonvisualized primary lesions of gastric cancer on preoperative MDCT. AJR Am J Roentgenol. 2007; 189:W315–W319. PMID: 18029842.
Article15. Park KJ, Lee MW, Koo JH, Park Y, Kim H, Choi D, et al. Detection of early gastric cancer using hydro-stomach CT: blinded vs unblinded analysis. World J Gastroenterol. 2011; 17:1051–1057. PMID: 21448358.
Article16. Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005; 241:27–39. PMID: 15621988.17. Bozzetti F, Bonfanti G, Bufalino R, Menotti V, Persano S, Andreola S, et al. Adequacy of margins of resection in gastrectomy for cancer. Ann Surg. 1982; 196:685–690. PMID: 7149820.
Article18. Wang SY, Yeh CN, Lee HL, Liu YY, Chao TC, Hwang TL, et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann Surg Oncol. 2009; 16:2738–2743. PMID: 19636636.
Article19. Jeong SH, Bae K, Ha CY, Lee YJ, Lee OJ, Jung WT, et al. Effectiveness of endoscopic clipping and computed tomography gastroscopy for the preoperative localization of gastric cancer. J Korean Surg Soc. 2013; 84:80–87. PMID: 23396626.
Article20. Choi JI, Joo I, Lee JM. State-of-the-art preoperative staging of gastric cancer by MDCT and magnetic resonance imaging. World J Gastroenterol. 2014; 20:4546–4557. PMID: 24782607.
Article21. Kumano S, Okada M, Shimono T, Kuwabara M, Yagyu Y, Imaoka I, et al. T-staging of gastric cancer of air-filling multidetector-row CT: comparison with hydro-multidetector-row CT. Eur J Radiol. 2012; 81:2953–2960. PMID: 22304982.
Article22. Furukawa K, Miyahara R, Itoh A, Ohmiya N, Hirooka Y, Mori K, et al. Diagnosis of the invasion depth of gastric cancer using MDCT with virtual gastroscopy: comparison with staging with endoscopic ultrasound. AJR Am J Roentgenol. 2011; 197:867–875. PMID: 21940574.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Computed Tomography Gastrography in the Surgical Management of a Gastric GIST
- Preoperative and intraoperative gastric tumor localization
- Usefullness of CT Gastrography and Vurtual Gastroscopy using Computed Tomography in Detection of Gastric Cancer
- Measurement of the Mucosal Surface Distance in the Early Gastric Cancer Using CT Gastrography
- Three-dimensional imaging modalities in endodontics