Clin Exp Vaccine Res.
2015 Jan;4(1):46-53. 10.7774/cevr.2015.4.1.46.
Clinical vaccine development
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, The Catholic University of Korea, Seoul, Korea. waystolove@catholic.ac.kr
- 2Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2049106
- DOI: http://doi.org/10.7774/cevr.2015.4.1.46
Abstract
- Vaccination is regarded as one of the biggest triumphs in the history of medicine. We are living in the most successful period of vaccine development. The accumulation of multidisciplinary knowledge and the investment of massive funding have enabled the development of vaccines against many infectious diseases as well as other diseases including malignant tumors. The paradigm of clinical vaccine evaluation and licensure has also been modernized based on scientific improvements and historical experience. However, there remain a number of hurdles to overcome. Continuous efforts are focused on increasing the efficacy and reducing the risks related to vaccine use. Cutting-edge knowledge about immunology and microbiology is being rapidly translated to vaccine development. Thus, physicians and others involved in the clinical development of vaccines should have sufficient understanding of the recent developmental trends in vaccination and the diseases of interest.
Keyword
MeSH Terms
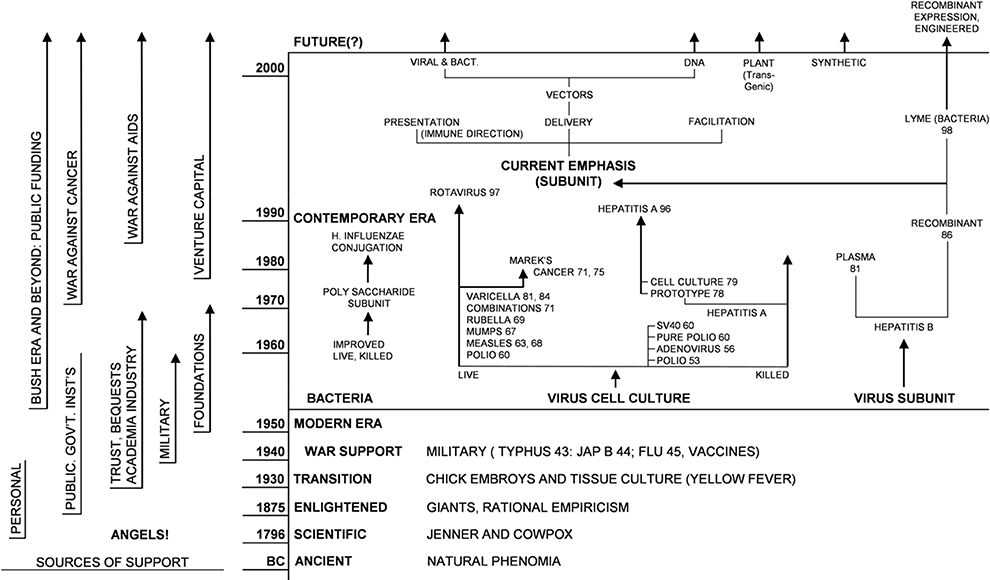
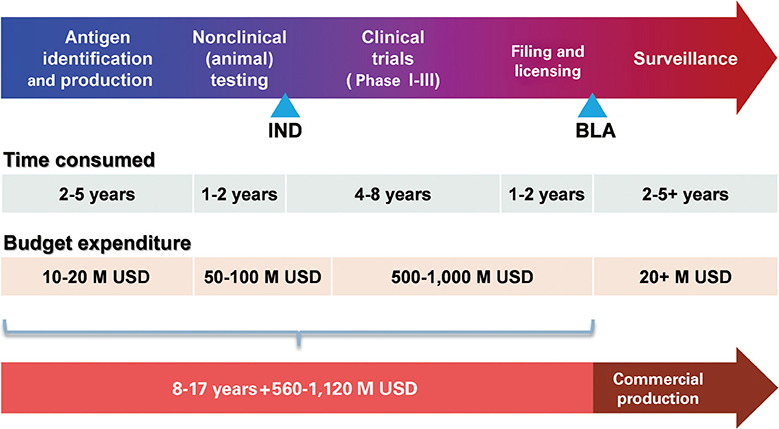
Figure
Reference
-
1. Hilleman MR. Vaccines in historic evolution and perspective: a narrative of vaccine discoveries. J Hum Virol. 2000; 3:63–76.
Article2. Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006; 12:9–14.
Article3. Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science. 2000; 287:443–449.
Article4. Gostin LO, Lucey D, Phelan A. The Ebola epidemic: a global health emergency. JAMA. 2014; 312:1095–1096.5. Leung AK. "Variolation" and vaccination in late Imperial China, Ca 1570-1911. In : Plotkin SA, editor. History of vaccine development. New York: Springer;2011. p. 5–12.6. Plotkin SA. Introduction. In : Plotkin SA, editor. History of vaccine development. New York: Springer;2011. p. 1–4.7. Wever PC, van Bergen L. Prevention of tetanus during the First World War. Med Humanit. 2012; 38:78–82.
Article8. Malito E, Rappuoli R. History of diphtheria vaccine development. In : Burkovski A, editor. Corynebacterium diphtheriae and related toxigenic species: genomics, pathogenicity and applications. Dordrecht: Springer;2014. p. 225–238.9. Lyons A, Longfield J, Kuschner R, et al. A double-blind, placebo-controlled study of the safety and immunogenicity of live, oral type 4 and type 7 adenovirus vaccines in adults. Vaccine. 2008; 26:2890–2898.
Article10. Hoyt K. Vaccine innovation: lessons from World War II. J Public Health Policy. 2006; 27:38–57.
Article11. Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nat Rev Microbiol. 2011; 9:889–893.
Article12. Hilleman MR. The development of live attenuated mumps virus vaccine in historic perspective and its role in the evolution of combined measles-mumps-rubella. In : Plotkin SA, editor. History of vaccine development. New York: Springer;2011. p. 207–218.13. Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins: V. The immunological specifity of an antigen prepared by combining the capsular polysaccharide of type Iii Pneumococcus with foreign protein. J Exp Med. 1931; 54:437–447.14. Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2012; 31:501–508.
Article15. Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010; 362:1511–1520.
Article16. Adkins JC, Wagstaff AJ. Recombinant hepatitis B vaccine: a review of its immunogenicity and protective efficacy against hepatitis B. BioDrugs. 1998; 10:137–158.17. Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991; 185:251–257.
Article18. Goetz KB, Pfleiderer M, Schneider CK. First-in-human clinical trials with vaccines: what regulators want. Nat Biotechnol. 2010; 28:910–916.
Article19. Vaccine development, testing, and regulation [Internet]. Philadelphia: The College of Physicians of Philadelphia;2014. cited 2014 Nov 1. Available from: http://www.historyofvaccines.org/content/articles/vaccine-development-testing-and-regulation.20. Different types of vaccines [Internet]. Philadelphia: The College of Physicians of Philadelphia;2014. cited 2014 Nov 1. Available from: http://www.historyofvaccines.org/content/articles/different-types-vaccines.21. World Health Organization. Guidelines on clinical evaluation of vaccines: regulatory expectations [Internet]. Geneva: World Health Organization;2004. cited 2014 Nov 2. Available from: http://www.who.int/biologicals/publications/trs/areas/vaccines/clinical_evaluation/en/.22. Committee for Human Medicinal Products (CHMP) of European Medicines Agency (EMA). Note for guidance on the clinical evaluation of vaccines. EMA/CHMP/VWP/164653/2005. London: European Medicines Agency;2005.23. U.S. Food and Drug Administration. Vaccine product approval process [Internet]. Silver Spring: U.S. Food and Drug Administration;2014. cited 2014 Nov 2. Available from: http://www.fda.gov/BiologicsBloodVaccines/DevelopmentApprovalProcess/BiologicsLicenseApplicationsBLAProcess/ucm133096.htm.24. U.S. Food and Drug Administration. Vaccine adverse events [Internet]. Silver Spring: U.S. Food and Drug Administration;2014. cited 2014 Nov 2. Available from: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/VaccineAdverseEvents/.25. Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011; 11:865–872.
Article26. Rappuoli R, Medaglini D. Big science for vaccine development. Vaccine. 2014; 32:4705–4707.
Article27. Thomas RE, Lorenzetti DL, Spragins W. Mortality and morbidity among military personnel and civilians during the 1930s and World War II from transmission of hepatitis during yellow fever vaccination: systematic review. Am J Public Health. 2013; 103:e16–e29.
Article28. Rappuoli R. Twenty-first century vaccines. Philos Trans R Soc Lond B Biol Sci. 2011; 366:2756–2758.
Article29. Donati C, Rappuoli R. Reverse vaccinology in the 21st century: improvements over the original design. Ann N Y Acad Sci. 2013; 1285:115–132.
Article30. Ovsyannikova IG, Poland GA. Vaccinomics: current findings, challenges and novel approaches for vaccine development. AAPS J. 2011; 13:438–444.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent update in HIV vaccine development
- COVID-19 vaccines development in Africa: a review of current situation and existing challenges of vaccine production
- Adenovirus Vectors: Excellent Tools for Vaccine Development
- Towards Vaccine 3.0: new era opened in vaccine research and industry
- Vaccines development in India: advances, regulation, and challenges