Anat Cell Biol.
2013 Dec;46(4):285-290. 10.5115/acb.2013.46.4.285.
Histomorphological and morphometrical changes of placental terminal villi of normotensive and preeclamptic mothers
- Affiliations
-
- 1Department of Anatomy, Narayana Medical College, Nellore, Andhra Pradesh, India. lesanshar@gmail.com
- 2Department of Biochemistry, Narayana Medical College, Nellore, Andhra Pradesh, India.
- 3Department of Anatomy, Vishnu Dental College, Bhimavaram, Andhra Pradesh, India.
- 4Department of Pathology, Alluri Sitaramaraju Academy of Medical Sciences, Eluru, Andhra Pradesh, India.
- KMID: 2046769
- DOI: http://doi.org/10.5115/acb.2013.46.4.285
Abstract
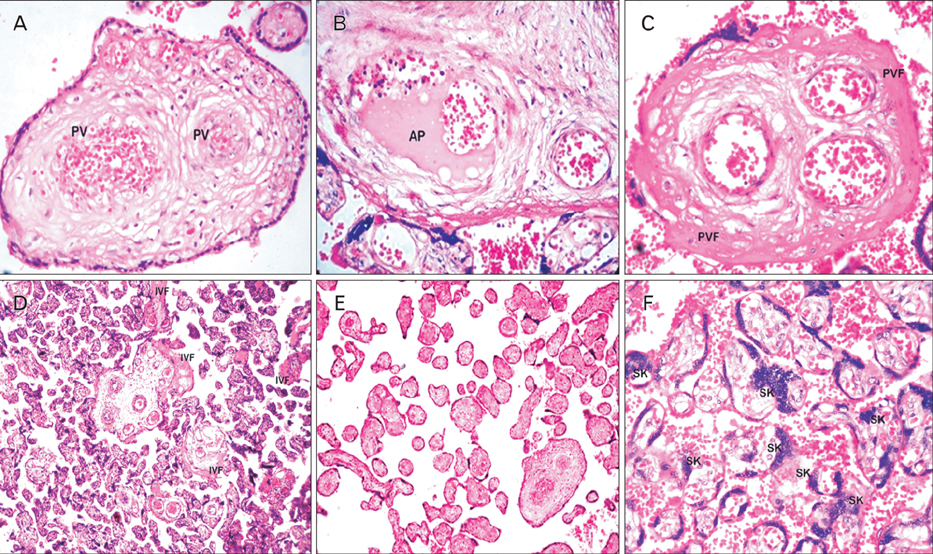
- Placental morphology and cellular arrangement are altered in maternal diseases such as preeclampsia (PE) in which oxygen delivery from the mother to the fetus is greatly disturbed, ultimately resulting in cellular oxidative stress. The present study was conducted at the Department of Anatomy and included 112 placentas (56 each from mothers with and without PE [controls]) collected at the Department of Obstetrics and Gynecology. A histological study was performed using hematoxylin and eosin staining. The morphology of stem and terminal villi (TV) was studied, and the surface area and diameter of TV and capillaries were measured. The gross placental morphometrical study revealed that the mean placental weight, thickness, diameter, and surface area were significantly lower in placentas with PE than in controls. The histomorphometrical findings of the villous surface area and diameter were lower in placentas with PE, whereas the TV density was higher in placentas with PE than in controls, and the differences were significant (P<0.0001). In these TV, the diameter and density of fetal blood vessels of placentas with PE were significantly lower than those of controls (P<0.05). In conclusion, the both morphological and histological changes in PE placentas are indicative of the pathogenesis of maternal and fetal morbidity and mortality in women with PE. The observed and comparative histomorphometrical changes indicate a decline in all aspects of the PE placenta, except the number of TV.
Keyword
MeSH Terms
Figure
Reference
-
1. Mardi K, Sharma J. Histopathological evaluation of placentas in IUGR pregnancies. Indian J Pathol Microbiol. 2003; 46:551–554.2. Vogel P. The current molecular phylogeny of Eutherian mammals challenges previous interpretations of placental evolution. Placenta. 2005; 26:591–596.3. Pardo F, Arroyo P, Salomón C, Westermeier F, Guzmán-Gutiérrez E, Leiva A, Sobrevia L. Gestational diabetes mellitus and the role of adenosine in the human placental endothelium and central nervous system. J Diabetes Metab. 2012; S2:010.4. Barker DJ, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006; 2:700–707.5. Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010; 54:525–530.6. Dhananjay BS, Dayananda G, Sendilkumaran D, Murthy N. A study of factors affecting perinatal mortality in eclampsia. J Physiol Biomed Sci. 2009; 22:2–5.7. Sankar KD, Bhanu PS, Kiran S, Ramakrishna BA, Shanthi V. Vasculosyncytial membrane in relation to syncytial knots complicates the placenta in preeclampsia: a histomorphometrical study. Anat Cell Biol. 2012; 45:86–91.8. Kishwara S, Ara S, Rayhan KA, Begum M. Morphological changes of placenta in preeclampsia. Bangladesh J Anat. 2009; 7:49–54.9. Akhlaq M, Nagi AH, Yousaf AW. Placental morphology in pre-eclampsia and eclampsia and the likely role of NK cells. Indian J Pathol Microbiol. 2012; 55:17–21.10. Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006; 27:141–169.11. Bdolah Y, Karumanchi SA, Sachs BP. Recent advances in understanding of preeclampsia. Croat Med J. 2005; 46:728–736.12. Huppertz B. Placental villous trophoblast: the altered balance between proliferation and apoptosis triggers pre-eclampsia. J Reprod Med Endocrinol. 2006; 3:103–108.13. Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003; 162:637–643.14. Dash PR, Whitley GS, Ayling LJ, Johnstone AP, Cartwright JE. Trophoblast apoptosis is inhibited by hepatocyte growth factor through the Akt and beta-catenin mediated up-regulation of inducible nitric oxide synthase. Cell Signal. 2005; 17:571–580.15. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003; 69:1–7.16. Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010; 140:803–813.17. Zigić Z, Marković S, Grbesa D, Ramić S, Halilović A. Quantitative research of capillaries in terminal villi of mature placentae. Bosn J Basic Med Sci. 2010; 10:147–152.18. Gill JS, Salafia CM, Grebenkov D, Vvedensky DD. Modeling oxygen transport in human placental terminal villi. J Theor Biol. 2011; 291:33–41.19. Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012; 5:9–18.20. Ilie R, Ilie C, Enătescu I, Bernad E, Frandes CD, Herbeck R. Histological modifications of the feto-placental interface in pregnancy induced hypertension. J Pediatr. 2011; 14:55–56.21. ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002; 77:67–75.22. Sivarao S, Vidyadaran MK, Jammal AB, Zainab S, Goh YM, Ramesh KN. Weight, volume and surface area of placenta of normal pregnant women and their relation to maternal and neonatal parameters in Malay, Chinese and Indian ethnic groups. Placenta. 2002; 23:691–696.23. Elias H, Henning A. Stereology of the human renal glomerulus. In : Weibel ER, Elias H, editors. Quantitative Methods in Morphology. Berlin: Springer-Verlag;1967. p. 155–158.24. Palkovts M, Fischen J. Karyometric investigations. Ch III. Budapest: Akademiai;1968. p. 75.25. Elias H, Hyde DM. An elementary introduction to stereology (quantitative microscopy). Am J Anat. 1980; 159:412–446.26. Myatt L. Role of placenta in preeclampsia. Endocrine. 2002; 19:103–111.27. Bokhari ZH, Khalid A, Tazeen N, Bukhari MH. Histomorphometric study of maternal side of placenta in preeclampsia. Ann King Edward Med Univ. 2010; 16:209–214.28. Kaufmann P, Burton G. Anatomy and genesis of the placenta. In : Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press;1994. p. 441–483.29. Mayhew TM. Villous trophoblast of human placenta: a coherent view of its turnover, repair and contributions to villous development and maturation. Histol Histopathol. 2001; 16:1213–1224.30. Burton GJ, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol. 2010; 54:303–312.31. Saeed I, Iqbal I, Sarfaraz R, Qamar K, Butt SA, Shaukat S. Histomorphological changes in placentae of preeclamtic mothers with reference to vasculosnycytial membrane thickness and syncytial knot formation. J Rawalpindi Med Coll. 2012; 16:51–54.32. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006; 27:939–958.33. Peng M, Yu L, Ding YL, Zhou CJ. Trophoblast cells invaing the placenta bed and change of spiral arteries and microvessels in pre-eclampsia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008; 33:121–129.34. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009; 30:473–482.35. Benirschke K, Kaufmann P. Pathology of the human placenta. 4th ed. New York: Springer Verlag;2000.36. Mayhew TM. Fetoplacental angiogenesis during gestation is biphasic, longitudinal and occurs by proliferation and remodelling of vascular endothelial cells. Placenta. 2002; 23:742–750.37. Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008; 61:1254–1260.38. Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000; 92:35–43.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Expression of Placental Nitric Oxide Synthase in Preeclampsia and Normal Pregnancy
- Immunohistochemical Localization of Endothelial Nitric Oxide Synthase in Normotensive and Preeclamptic Placentas
- The nitric oxide synthase activity and expression in human placenta from preeclamptic pregnancies
- The effect of ginsenoside Rk1 in junctional protein of severe preeclamptic placenta
- Expression of alpha-smooth muscle actin and collagenIV in villous stroma of placental terminal villi and congestion fetal capillary in growth restricted pregnancies with severe preeclampsia