J Korean Assoc Oral Maxillofac Surg.
2012 Jun;38(3):145-151. 10.5125/jkaoms.2012.38.3.145.
Expression of endoglin and podoplanin in early and advanced oral squamous cell carcinoma
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Gangneung-Wonju National University, Gangneung, Korea. ywpark@gwnu.ac.kr
- KMID: 2043843
- DOI: http://doi.org/10.5125/jkaoms.2012.38.3.145
Abstract
OBJECTIVES
Angiogenesis and lymphangiogenesis are correlated with tumor growth and lymph node metastasis in cases of oral squamous cell carcinoma (OSCC). Endoglin is one of the representative vascular endothelial cell markers. Podoplanin is also a representative marker used in order to detect lymphatic endothelial cells. The aim of this study was to determine the correlation between the expression of endoglin/podoplanin and clinical variables associated with OSCC progression.
MATERIALS AND METHODS
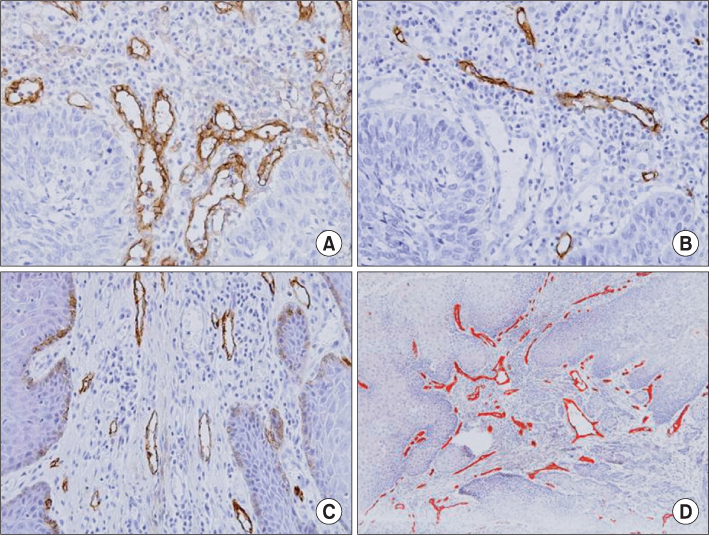
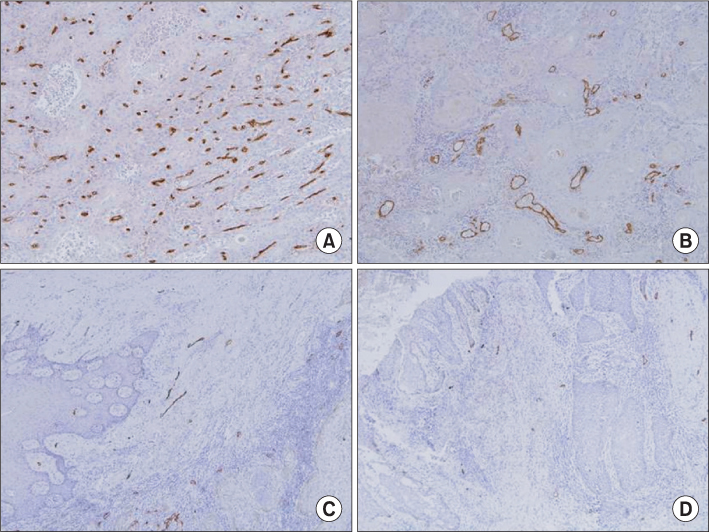
Paraffin embedded tissue specimens from 21 patients diagnosed with OSCC were used in this study. Ten patients were diagnosed with early clinical stage (I or II) and 11 patients with advanced clinical stage (III or IV) OSCC. Five patients had positive lymph node involvement. Primary antibodies for endoglin and podoplanin were used to perform the immunohistochemical detection of the vascular and lymphatic endothelial cells. The expression of endoglin and podoplanin was examined by an image analysis program in the three most highly expressed regions of each specimen.
RESULTS
The average endoglin expression was observed to be 1.691+/-0.920 in the advanced stage (III, IV) specimens and 0.797+/-0.583 in the early stage (I, II) specimens (P=0.020). The average expression of podoplanin was 0.286+/-0.228 in the advance stage (III, IV) specimens and 0.374+/-0.157 in the early stage (I, II) specimens (P>0.05). There was no statistically significant difference in the expression of endoglin and podoplanin, regardless of whether or not the lymph node was positive.
CONCLUSION
The expression of endoglin was significantly higher in the advanced stage specimens than that in the early stage specimens. Therefore, we concluded that endoglin is a useful molecular marker for use in the evaluation of the progression of OSCC.
Keyword
MeSH Terms
Figure
Reference
-
1. McDowell JD. An overview of epidemiology and common risk factors for oral squamous cell carcinoma. Otolaryngol Clin North Am. 2006. 39:277–294.
Article2. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma 2: chromosomal aberrations. Oral Oncol. 2000. 36:311–327.
Article3. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral Oncol. 2000. 36:256–263.
Article4. Oliveira LR, Ribeiro-Silva A. Prognostic significance of immunohistochemical biomarkers in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2011. 40:298–307.
Article5. Scully C, Bagan JV. Recent advances in oral oncology 2008; squamous cell carcinoma imaging, treatment, prognostication and treatment outcomes. Oral Oncol. 2009. 45:e25–e30.
Article6. Massano J, Regateiro FS, Januário G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. 102:67–76.
Article7. van der Schroeff MP, Baatenburg de Jong RJ. Staging and prognosis in head and neck cancer. Oral Oncol. 2009. 45:356–360.
Article8. Kim JY, Rotaru H, Kim SG. The clinical significance of the expression of TGF-beta 1 and MMP-2 related to the regional lymph node metastasis in the oral squamous cell carcinoma. J Korean Assoc Oral Maxillofac Surg. 2007. 33:199–203.9. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000. 407:249–257.
Article10. Hasina R, Lingen MW. Angiogenesis in oral cancer. J Dent Educ. 2001. 65:1282–1290.
Article11. Palomba A, Gallo O, Brahimi A, Franchi A. Evaluation of lymphangiogenesis in premalignant conditions of the head and neck mucosa. Head Neck. 2010. 32:1681–1685.
Article12. Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009. 13:1405–1416.
Article13. Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003. 17:984–992.
Article14. Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008. 28:2997–3006.15. Kyzas PA, Agnantis NJ, Stefanou D. Endoglin (CD105) as a prognostic factor in head and neck squamous cell carcinoma. Virchows Arch. 2006. 448:768–775.
Article16. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991. 324:1–8.
Article17. Zhang Z, Helman JI, Li LJ. Lymphangiogenesis, lymphatic endothelial cells and lymphatic metastasis in head and neck cancer--a review of mechanisms. Int J Oral Sci. 2010. 2:5–14.
Article18. Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007. 25:4298–4307.
Article19. Tortora G, Melisi D, Ciardiello F. Angiogenesis: a target for cancer therapy. Curr Pharm Des. 2004. 10:11–26.
Article20. Dredge K, Dalgleish AG, Marriott JB. Angiogenesis inhibitors in cancer therapy. Curr Opin Investig Drugs. 2003. 4:667–674.21. Madhusudan S, Harris AL. Drug inhibition of angiogenesis. Curr Opin Pharmacol. 2002. 2:403–414.
Article22. Rugo HS. Bevacizumab in the treatment of breast cancer: rationale and current data. Oncologist. 2004. 9:Suppl 1. 43–49.
Article23. Planchard D. Bevacizumab in non-small-cell lung cancer: a review. Expert Rev Anticancer Ther. 2011. 11:1163–1179.
Article24. Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess. 2007. 11:1–128.
Article25. Lastres P, Letamendía A, Zhang H, Rius C, Almendro N, Raab U, et al. Endoglin modulates cellular responses to TGF-beta 1. J Cell Biol. 1996. 133:1109–1121.
Article26. Quintanilla M, Ramirez JR, Pérez-Gómez E, Romero D, Velasco B, Letarte M, et al. Expression of the TGF-beta coreceptor endoglin in epidermal keratinocytes and its dual role in multistage mouse skin carcinogenesis. Oncogene. 2003. 22:5976–5985.
Article27. Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000. 217:42–53.
Article28. Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999. 284:1534–1537.
Article29. Schimming R, Reusch P, Kuschnierz J, Schmelzeisen R. Angiogenic factors in squamous cell carcinoma of the oral cavity: do they have prognostic relevance? J Craniomaxillofac Surg. 2004. 32:176–181.
Article30. Schimming R, Marmé D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head Neck. 2002. 24:151–156.
Article31. Eshghyar N, Mohammadi N, Rahrotaban S, Motahhary P, Vahedi Vaez SM. Endoglin (CD105) positive microvessel density and its relationship with lymph node metastasis in squamous cell carcinoma of the tongue. Arch Iran Med. 2011. 14:276–280.32. Chuang HC, Su CY, Huang HY, Chien CY, Chen CM, Huang CC. High expression of CD105 as a prognostic predictor of early tongue cancer. Laryngoscope. 2006. 116:1175–1179.
Article33. Wicki A, Christofori G. The potential role of podoplanin in tumour invasion. Br J Cancer. 2007. 96:1–5.
Article34. Funayama A, Cheng J, Maruyama S, Yamazaki M, Kobayashi T, Syafriadi M, et al. Enhanced expression of podoplanin in oral carcinomas in situ and squamous cell carcinomas. Pathobiology. 2011. 78:171–180.
Article35. Siriwardena BS, Kudo Y, Ogawa I, Udagama MN, Tilakaratne WM, Takata T. VEGF-C is associated with lymphatic status and invasion in oral cancer. J Clin Pathol. 2008. 61:103–108.
Article36. Zhang Z, Pan J, Li L, Wang Z, Xiao W, Li N. Survey of risk factors contributed to lymphatic metastasis in patients with oral tongue cancer by immunohistochemistry. J Oral Pathol Med. 2011. 40:127–134.
Article37. Okada Y. Relationships of cervical lymph node metastasis to histopathological malignancy grade, tumor angiogenesis, and lymphatic invasion in tongue cancer. Odontology. 2010. 98:153–159.
Article38. Zhao D, Pan J, Li XQ, Wang XY, Tang C, Xuan M. Intratumoral lymphangiogenesis in oral squamous cell carcinoma and its clinicopathological significance. J Oral Pathol Med. 2008. 37:616–625.
Article39. Longatto Filho A, Oliveira TG, Pinheiro C, de Carvalho MB, Curioni OA, Mercante AM, et al. How useful is the assessment of lymphatic vascular density in oral carcinoma prognosis? World J Surg Oncol. 2007. 5:140.
Article40. Miyahara M, Tanuma J, Sugihara K, Semba I. Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer. 2007. 110:1287–1294.
Article41. Kreppel M, Scheer M, Drebber U, Ritter L, Zöller JE. Impact of podoplanin expression in oral squamous cell carcinoma: clinical and histopathologic correlations. Virchows Arch. 2010. 456:473–482.
Article42. Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, et al. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006. 107:563–569.
Article43. Huber GF, Fritzsche FR, Züllig L, Storz M, Graf N, Haerle SK, et al. Podoplanin expression correlates with sentinel lymph node metastasis in early squamous cell carcinomas of the oral cavity and oropharynx. Int J Cancer. 2011. 129:1404–1409.
Article44. Kanner WA, Galgano MT, Atkins KA. Podoplanin expression in basal and myoepithelial cells: utility and potential pitfalls. Appl Immunohistochem Mol Morphol. 2010. 18:226–230.45. Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, et al. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer. 2006. 95:1611–1625.
Article46. Choi WW, Lewis MM, Lawson D, Yin-Goen Q, Birdsong GG, Cotsonis GA, et al. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol. 2005. 18:143–152.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic problem of squamous papilloma and oral mucosa malignancy
- Direct Contact with Platelets Induces Podoplanin Expression and Invasion in Human Oral Squamous Cell Carcinoma Cells
- Clinicopathologic parameters in predicting cervical nodal metastasis in early squamous cell carcinoma of the oral cavity
- Contrasting Roles of Different Endoglin Forms in Atherosclerosis
- Change of the Invasiveness with Selective Cox-2 Inhibition in an Oral Squamous Cell Carcinoma Cell Line, KB; Preliminary in Vitro Study