J Korean Soc Spine Surg.
2004 Mar;11(1):1-13. 10.4184/jkss.2004.11.1.1.
Effects of Polyphosphate on the Fusion of Rabbit Lumbar Spine
- Affiliations
-
- 1Department of Orthopaedic Surgery, St. Paul's Hospital, Kang-Nam St. Mary's Hospital, Korea. kyh@cuk.cmc.ac.kr
- 2Department of Orthopaedic Surgery, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- KMID: 2003186
- DOI: http://doi.org/10.4184/jkss.2004.11.1.1
Abstract
- STUDY DESIGN: Posterior and posterolateral fusions were performed in rabbit lumbar spines.
OBJECTIVES
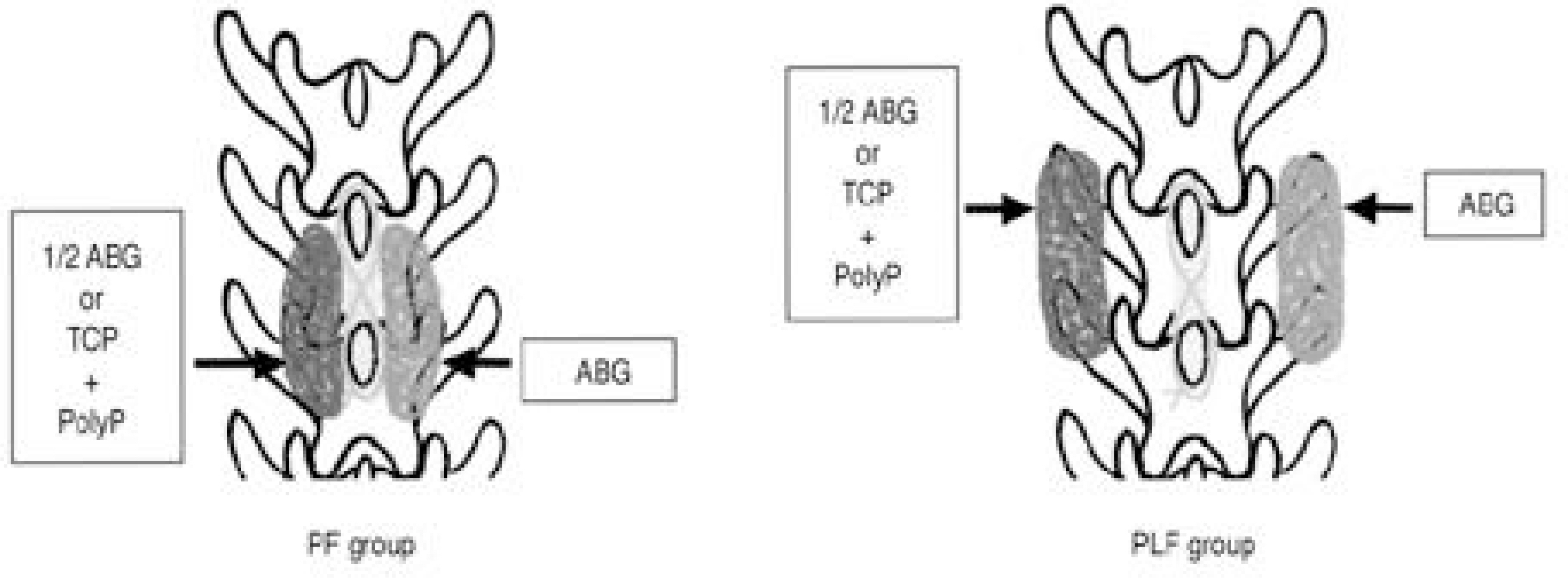
To investigate the osteoinductive effect of polyphosphates. SUMMARY AND LITERATURE REVIEW: Inorganic polyphosphates are known to be rich in osteoblasts and involved in the mineralization process in bone metabolism. However, no study has been undertaken to investigate the osteoinductive effect of polyphosphates. MATERIALS AND METHODS: Forty adult New Zealand white rabbits underwent monolevel lumbar fusions, and were divided into two groups according to the fusion beds: twenty each between the laminae (posterior fusion group, PF group) and between the transverse processes (posterolateral fusion group, PLF group). In ten of twenty rabbits in the PF group, 0.8gm of autogenous iliac bone was grafted onto the right sides of the laminae, which were used as a control group (C1), with 0.4gm autogenous bone immersed in polyphosphate solution in the left sides as an experimental group (E1). In the other ten, 0.8gm of autogenous bone was grafted onto the right sides (C2) and 0.8gm of tricalcium phosphate porous blocks containing polyphosphate in the left sides (E2). The other twenty rabbits of the PLF group were similarly divided into C1, E1, C2 and E2 groups by grafting the same amount of materials between the transverse processes. The animals were sacrificed at the 16th postoperative week and the fusions evaluated grossly, radiologically and histologically. Statistical differences between the groups (C1 vs. E1, C2 vs. E2 and E1 vs. E2) in each of the PF and PLF groups were compared by chi-square tests.
RESULTS
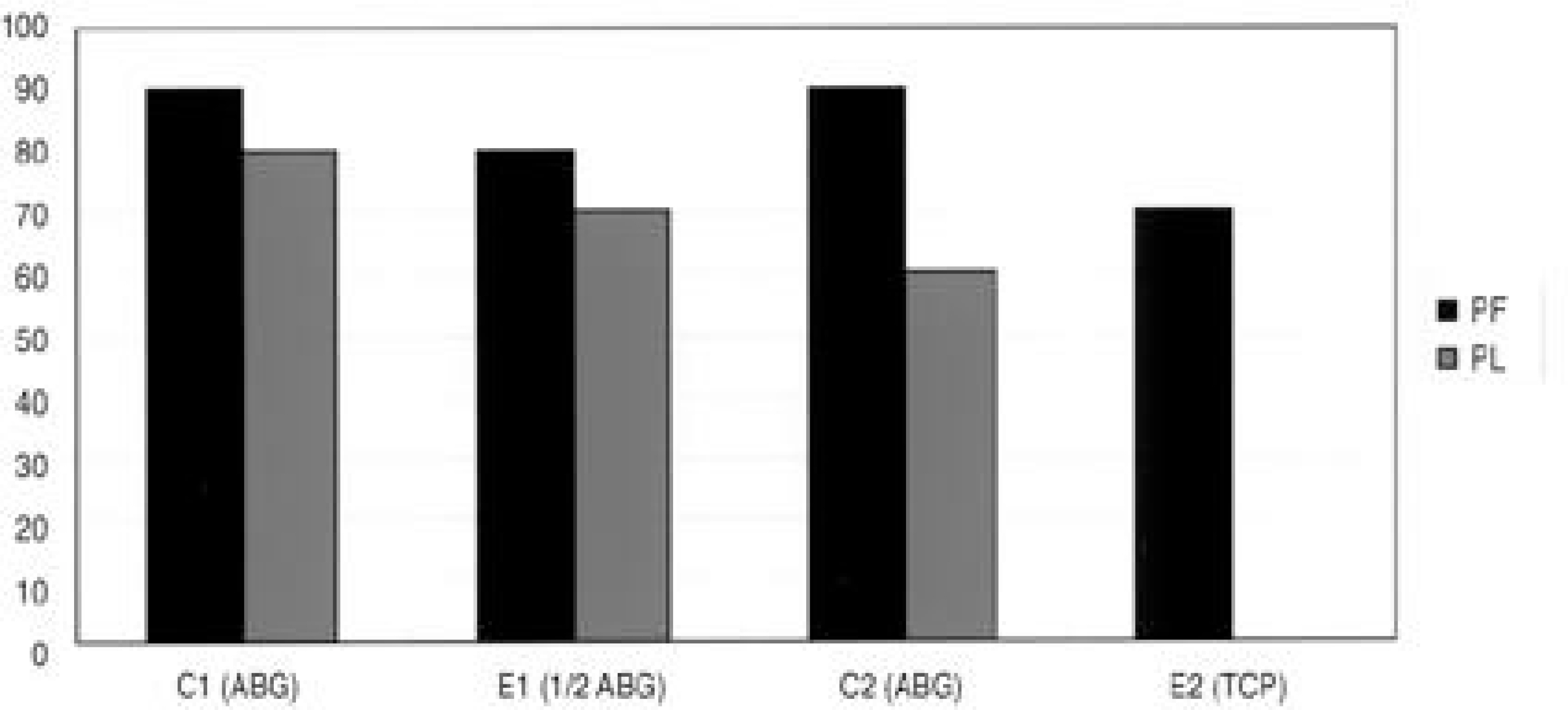
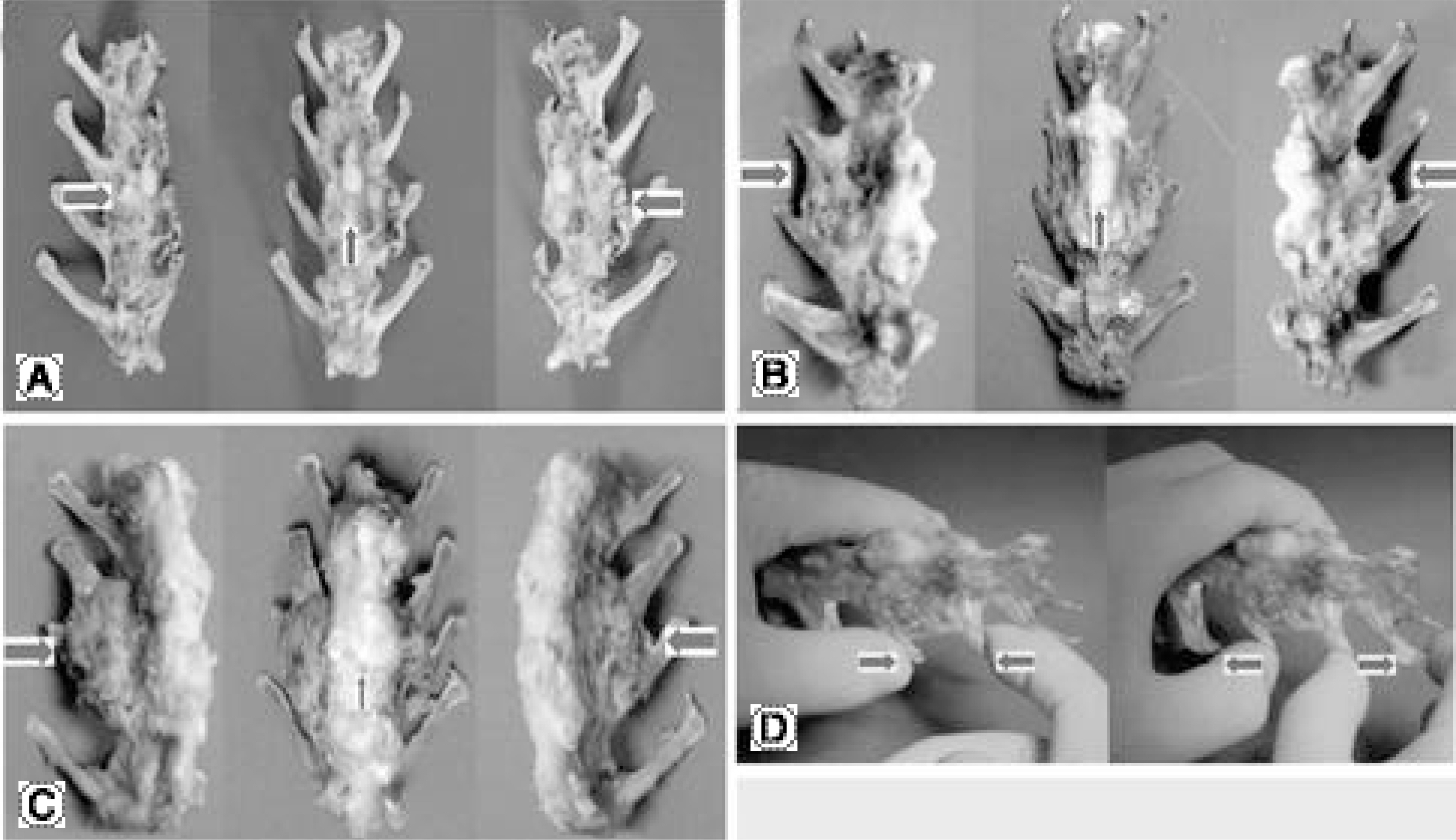
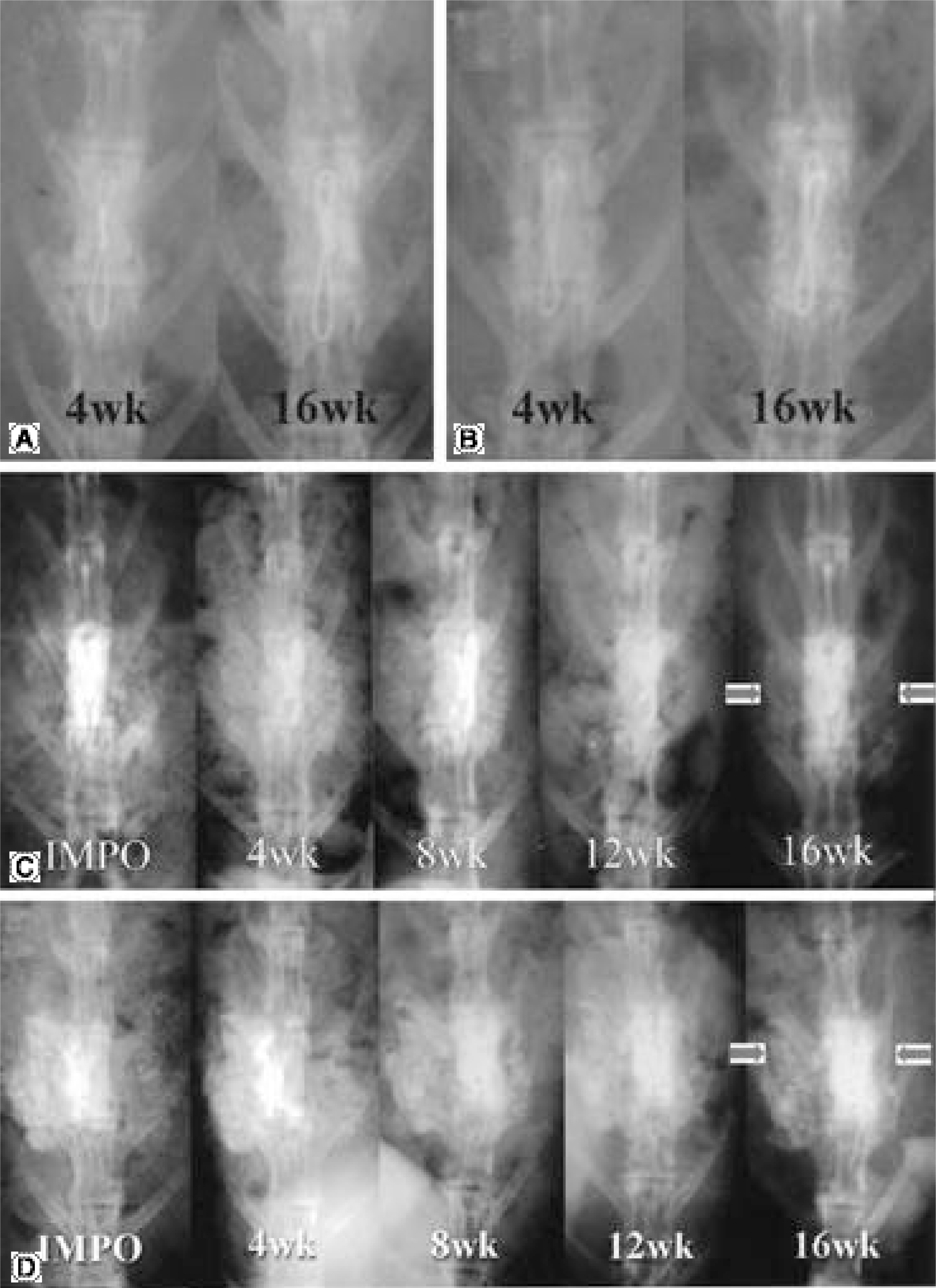
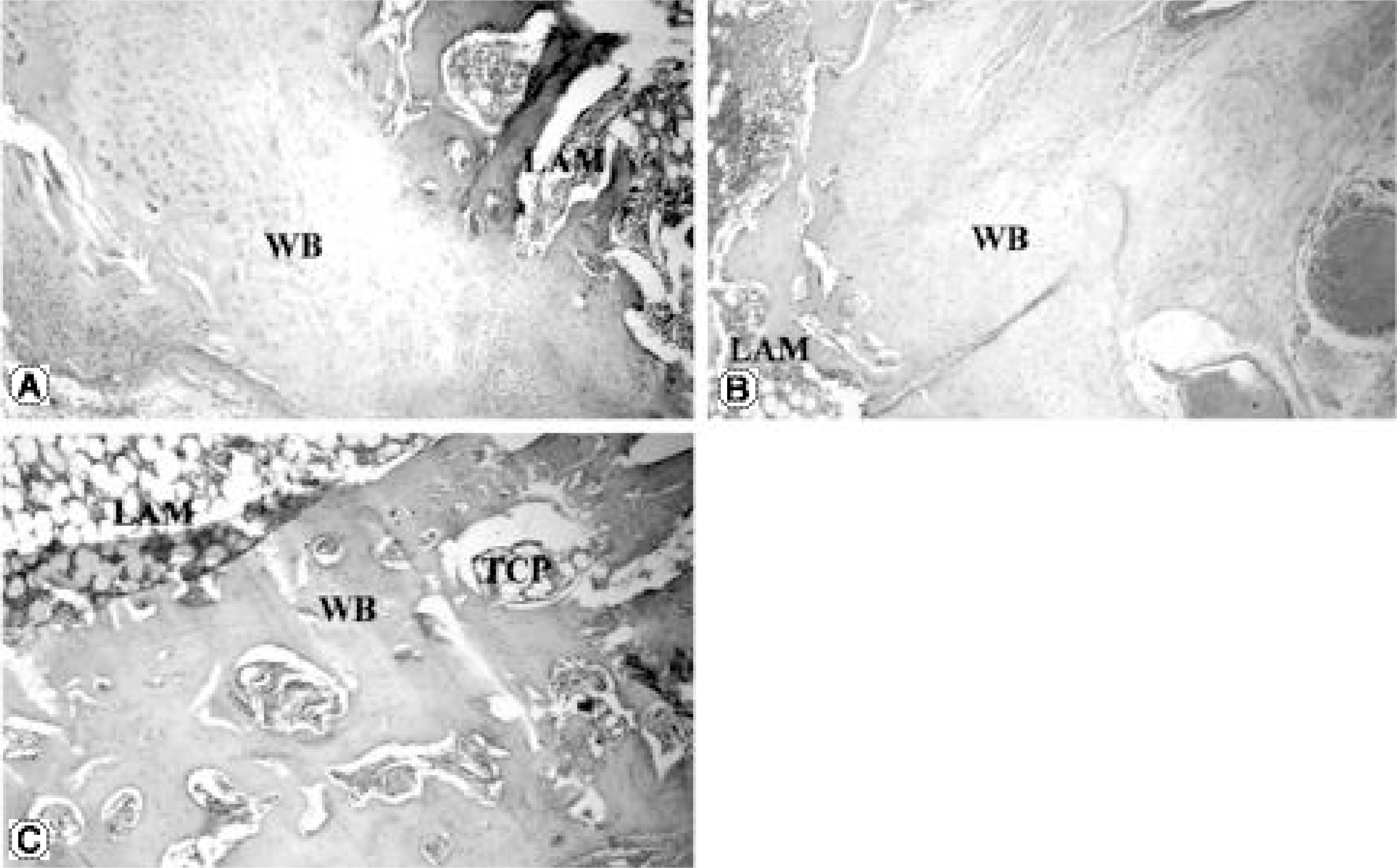
The fusions were finally determined by the gross finding using manual palpation. In the PF group, bony fusions were obtained in 90, 80, 90 and 70% of the C1, E1, C2 and E2 groups, respectively. In the PLF group, these were 80, 70, 60 and 0% of the C1, E1, C2 and E2 groups, respectively. Statistical analysis revealed differences only between C2 and E2 (p=0.005), and between E1 and E2 (p=0.002) of the PLF group. Histologically, beta-tricalcium phosphate particles containing polyphosphate were transformed into the osteoid in some areas of the PLF-E2 group, although only fibrous unions were obtained grossly.
CONCLUSIONS
It is suggested that the polyphosphate may have an osteoinductive effect, even though the osteoinductive potency was very week in this fusion model of the rabbit lumbar spine. Therefore, further explorations, such as the threshold and optimal concentrations of polyphosphate in vivo and the best carrier material of polyphosphate, should be performed to obtain the optimal conditions for fusion.
MeSH Terms
Figure
Reference
-
1). Boden SD, Andersson GBJ, Anderson DG, et al. Overview of bone morphogenetic proteins for spine fusion, Spine. 2002; 15:S1.2). Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: A prospective, randomized clinical pilot trial. Spine. 2002; 27:2662–2673.3). Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine. 2002; 15:S26–31.
Article4). Boden SD, Schimandle JH, Hutton WC, Chen MI. The use of an osteoinductive growth factor for lumbar spinal fusion. Part I: Biology of spinal fusion. Spine. 1995; 20:2626–2632.5). Boden SD, Schimandle JH, Hutton WC. The use of an osteeoinductive growth factor for lumbar spinal fusion. Part II: Study of dose, carrier, and species. Spine. 1995; 20:2633–2644.6). De Groot K. Bioceramics consisting of calcium phosphate salts. Biomaterials. 1980; 1:47–50.
Article7). Delecrin J, Aguado E, NGuyen JM, Pyre D, Royer J, Passuti N. Influence of local environment on incorpo -ration of ceramic for lumbar fusion. Comparison of laminar and intertransverse sites in a canine model. Spine. 1997; 1:1683–1689.8). Delecrin J, Deschamps C, Romih M, Heymann D, Passuti N. Influence of bone environment on ceramic osteointegration in spinal fusion: comparison of bone-poor and bone-rich sites. Eur Spine J. 2001; 10:S110–113.9). Eggli PS, Muller W, Schenk RK. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin Orthop. 1988; 232:127–138.10). Fischgrund JS, James SB, Chabot MC, et al. Augmentation of autograft using rhBMP-2 and different carrier media in the canine spinal fusion model. J Spinal Disord. 1997; 10:467–472.
Article11). Grauer JN, Patel TC, Erulkar JS, Troiano NW, Panjabi MM, Friedlaender GE. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2001; 26:127–133.
Article12). Ha KY, Roh SH. Tumor Necrosis Factor-α and resorption of calcium sulfate used as a bone graft substitute in spinal fusion in rabbits. J Kor Spine Surg. 2002; 37:115–122.
Article13). Ha KY. Biosynthetic graft as a bone graft substitute in spinal fusion. J Kor Spine Surg. 2000; 7:150–161.14). Ha KY, Han CW, Ryoo SJ. Intertransverse lumbar fuion using calcium sulfate as a bone graft substitute in rabbit. J Kor Spine Surg. 1999; 6:336–343.15). Ha KY, Park SJ, Choi WS, Roh SH. Calcium sulfate as a bone graft substitute for spinal fusion. J Kor Spine Surg. 2001; 8:53–61.16). Helm G, Anderson DG, Andersson GBJ, et al. Bo ne morphogenetic proteins, basic science. Spine. 2002; 27:S9.17). Holinger JO, Brekke J, Gruskin E, Lee D. Role of bone substitutes. Clin Orthop. 1996; 324:55–65.18). Hubbard WG. Physiological calcium phosphate as orthopedic implant. Diss Abstr Int. 1974; 35:1683B.19). Kandziora F, Schmidmaier G, Schollmeier G, Bail H, Pflugmacher R, Gorke T, Wagner M, Raschke M, Mit-tlmeier T, Haas NP. IGF-I and TGF-beta1 application by a poly-(D,L-Lactide)-coated cage promotes intervertebral bone matrix formation in the sheep cervical spine. Spine. 2002; 27:1710–1723.20). Kim HY. Personal communication.21). Kitsugi T, Yamamuro T, Nakamura T, Kotani S, Kokubo T, Takeuchi H. Four calcium phosphate ceramics as bone substitutes for non-weight-bearing. Biomaterials. 1993; 14:216–224.
Article22). Klein CP, Driessen AA, de Groot K, van den Hooff A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mater Res. 1983; 17:769–784.
Article23). Konishi S, Nakamura H, Seki M, Nagayama R, Yamano Y. Hydroxyapatite granule graft combined with recombinant human bone morphogenic protein-2 for solid lumbar fusion. J Spinal Disord. 2002; 15:237–244.
Article24). Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995; 177:491–496.
Article25). Kornberg A, Rao NN, Ault-Riche D. I norga n ic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999; 68:89–125.26). Leyhausen G, Lorenz B, Zhu H, et al. I n o r g a n i c polyphosphate in human osteblast-like cells. J Bone Miner Res. 1998; 13:803–812.27). Manjubala I, Sivakumar M, Sureshkumar RV, Sastry TP. Bioactivity and osseointegration study of calcium phosphate ceramic of different chemical composition. J Biomed Mater Res. 2002; 63:200–208.
Article28). Minamide A, Kawakami M, Hashizume H, Sakata R, Tamaki T. Evaluation of carriers of bone morphogenetic protein for spinal fusion. Spine. 2001; 26:933–939.
Article29). Ogose A, Hotta T, Hatano H, et al. Histological exami -nation of beta-tricalcium phosphate graft in human femur. J Biomed Mater Res. 2002; 63:601–604.30). Schroeder HC, Kuerz L, Mueller WE, Lorenz B. Polyphosphate in bone. Biochemistry. 2000; 65:296–303.31). Spivak JM, Hasharoni A. Use of hydroxyapatite in spine surgery. Eur Spine J. 2001; 10:S197–204.
Article32). Summer BN, Eisenstein SM. Donor site pain from the ilium: A complication of lumbar spine fusion. J Bone Joint Surg. 1989; 71-B:667–680.33). Urist MR. Bone formation by autoinduction. Science. 1965; 150:893–899.
Article34). Young EM, Chapman MW. Morbidity at bone graft sites. J Orthop Trauma. 1989; 3:192–195.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Principles of Lumbar Spine Stabilization
- Biportal Endoscopic Transforaminal Lumbar Interbody Fusion with Percutaneous Instrumentation: A Technical Note
- Histomorphometric study on effect of the polyphosphate for bone regeneration
- Letter: Comparison of Posterior Lumbar Interbody Fusion and Posterolateral Lumbar Fusion in Monosegmental Vacuum Phenomenon within an Intervertebral Disc
- Does the Cage Position in Transforaminal Lumbar Interbody Fusion Determine Unilateral versus Bilateral Screw Placement?: A Review of the Literature