J Korean Soc Radiol.
2013 Nov;69(5):377-384. 10.3348/jksr.2013.69.5.377.
Factors Influencing Liver and Spleen Volume Changes after Donor Hepatectomy for Living Donor Liver Transplantation
- Affiliations
-
- 1Department of Radiology, Kyungpook National University Hospital, Daegu, Korea. hkryeom@knu.ac.kr
- 2Department of Occupational Medicine, Kyungpook National University Hospital, Daegu, Korea.
- KMID: 2002859
- DOI: http://doi.org/10.3348/jksr.2013.69.5.377
Abstract
- PURPOSE
To define the changes in liver and spleen volumes in the early postoperative period after partial liver donation for living-donor liver transplantation (LDLT) and to determine factors that influence liver and spleen volume changes.
MATERIALS AND METHODS
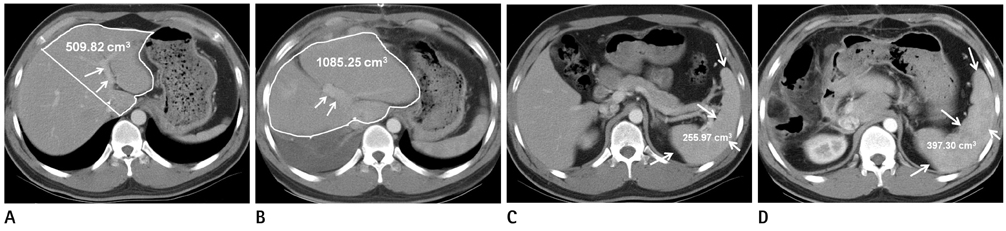
27 donors who underwent partial hepatectomy for LDLT were included in this study. The rates of liver and spleen volume change, measured with CT volumetry, were correlated with several factors. The analyzed factors included the indocyanine green (ICG) retention rate at 15 minutes after ICG administration, preoperative platelet count, preoperative liver and splenic volumes, resected liver volume, resected-to-whole liver volume ratio (LV(R)/LV(W)), resected liver volume to the sum of whole liver and spleen volume ratio [LV(R)/(LV(W) + SV(0))], and pre and post hepatectomy portal venous pressures.
RESULTS
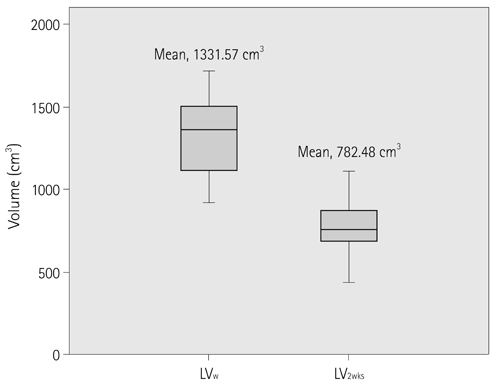
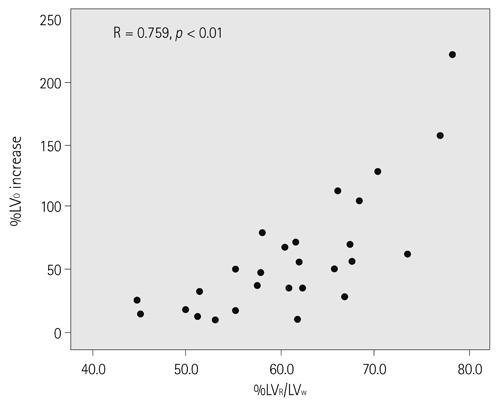
In all hepatectomy donors, the volumes of the remnant liver and spleen were increased (increased rates, 59.5 +/- 50.5%, 47.9 +/- 22.6%). The increment rate of the remnant liver volume revealed a positive correlation with LV(R)/LV(W) (r = 0.759, p < 0.01). The other analyzed factors showed no correlation with changes in liver and spleen volumes.
CONCLUSION
The spleen and remnant liver volumes were increased at CT volumetry performed 2 weeks after partial liver donation. Among the various analyzed factors, LV(R)/LV(W) influences the increment rate of the remnant liver volume.
MeSH Terms
Figure
Reference
-
1. Nadalin S, Bockhorn M, Malagó M, Valentin-Gamazo C, Frilling A, Broelsch CE. Living donor liver transplantation. HPB (Oxford). 2006; 8:10–21.2. Hardy KJ. Liver surgery: the past 2000 years. Aust N Z J Surg. 1990; 60:811–817.3. Bucher NL. Experimental aspects of hepatic regeneration. N Engl J Med. 1967; 277:686–696 contd.4. Fausto N. Liver regeneration: from laboratory to clinic. Liver Transpl. 2001; 7:835–844.5. Court FG, Wemyss-Holden SA, Dennison AR, Maddern GJ. The mystery of liver regeneration. Br J Surg. 2002; 89:1089–1095.6. Olthoff KM. Molecular pathways of regeneration and repair after liver transplantation. World J Surg. 2002; 26:831–837.7. Ibrahim S, Chen CL, Wang CC, Wang SH, Lin CC, Liu YW, et al. Liver regeneration and splenic enlargement in donors after living-donor liver transplantation. World J Surg. 2005; 29:1658–1666.8. Zollinger RM, Zollinger RM Jr. Left hepatic lobectomy. In : Zollinger RM, Zollinger RM, editors. Atlas of Surgical Operations. 4th ed. New York: Macmillian;1990. p. 172–175.9. Akimaru K, Onda M, Tajiri T, Yoshida H, Yokomuro S, Mamada Y, et al. Hypersplenism induced by hepatectomy. Hepatogastroenterology. 2001; 48:1170–1175.10. Ando H, Nagino M, Arai T, Nishio H, Nimura Y. Changes in splenic volume during liver regeneration. World J Surg. 2004; 28:977–981.11. Lemke AJ, Hosten N, Neumann K, Müller B, Neuhaus P, Felix R, et al. [CT volumetry of the liver before transplantation]. Rofo. 1997; 166:18–23.12. Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000; 69:1375–1379.13. Tanaka W, Yamanaka N, Oriyama T, Katoh T, Kuroda N, Okamoto E. Multivariate analysis of liver regenerative capacity after hepactectomy in humans. J Hep Bil Pancr Surg. 1997; 4:78–82.14. Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto J, et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology. 1993; 18:79–85.15. Greene AK, Wiener S, Puder M, Yoshida A, Shi B, Perez-Atayde AR, et al. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann Surg. 2003; 237:530–535.16. Nakagami M, Morimoto T, Itoh K, Arima Y, Yamamoto Y, Ikai I, et al. Patterns of restoration of remnant liver volume after graft harvesting in donors for living related liver transplantation. Transplant Proc. 1998; 30:195–199.17. Nagasue N, Yukaya H, Ogawa Y, Higashi T. Portal pressure following partial to extensive hepatic resection in patients with and without cirrhosis of the liver. Ann Chir Gynaecol. 1983; 72:18–22.18. Ueda S, Yamanoi A, Hishikawa Y, Dhar DK, Tachibana M, Nagasue N. Transforming growth factor-beta1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab Invest. 2003; 83:1595–1603.19. Kaido T, Oe H, Yoshikawa A, Okajima A, Imamura M. Expressions of molecules associated with hepatocyte growth factor activation after hepatectomy in liver cirrhosis. Hepatogastroenterology. 2004; 51:547–551.20. Rosenkranz E, Charters AC 3rd, Orloff MJ. Regeneration in rat liver injured by carbon tetrachloride. Surg Forum. 1975; 26:411–412.21. Tomiya T, Tani M, Yamada S, Hayashi S, Umeda N, Fujiwara K. Serum hepatocyte growth factor levels in hepatectomized and nonhepatectomized surgical patients. Gastroenterology. 1992; 103:1621–1624.22. Sato K, Tanaka M, Tanikawa K. The effect of spleen volume on liver regeneration after hepatectomy--a clinical study of liver and spleen volumes by computed tomography. Hepatogastroenterology. 1995; 42:961–965.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Donor Complication in Living Donor Liver Transplantation
- Laparoscopic living donor hepatectomy
- Minimal-incision donor right hepatectomy for living donor liver transplantation
- Decreasing the operation time of living donor liver transplantation in the era of laparoscopic living donor hepatectomy
- A Single Center Experience for a Feasibility of Totally Laparoscopic Living Donor Right Hepatectomy