J Korean Acad Prosthodont.
2014 Jan;52(1):9-17. 10.4047/jkap.2014.52.1.9.
On the osseointegration of zirconia and titanium implants installed at defect site filled with xenograft material
- Affiliations
-
- 1Department of Prosthodontics, College of Dentistry, Dankook University, Cheonan, Korea. cho8511@dankook.ac.kr
- KMID: 2000142
- DOI: http://doi.org/10.4047/jkap.2014.52.1.9
Abstract
- PURPOSE
The purpose of this study was to compare zirconia implants with titanium implants from the view point of biomechanical stability and histologic response on osseointegration when those were placed with xenograft materials.
MATERIALS AND METHODS
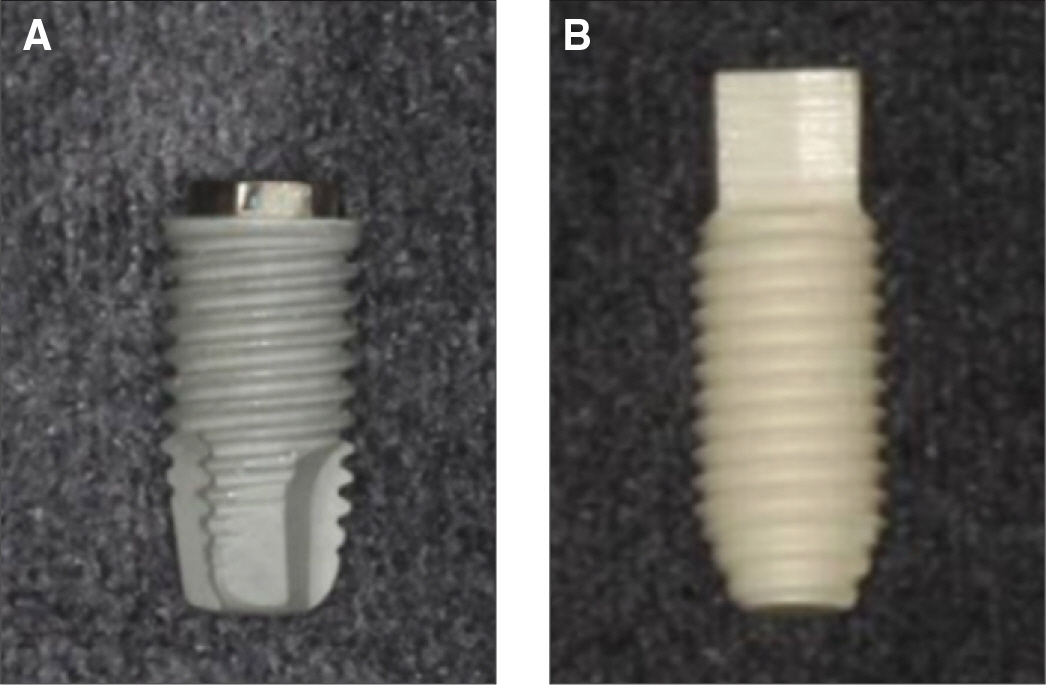
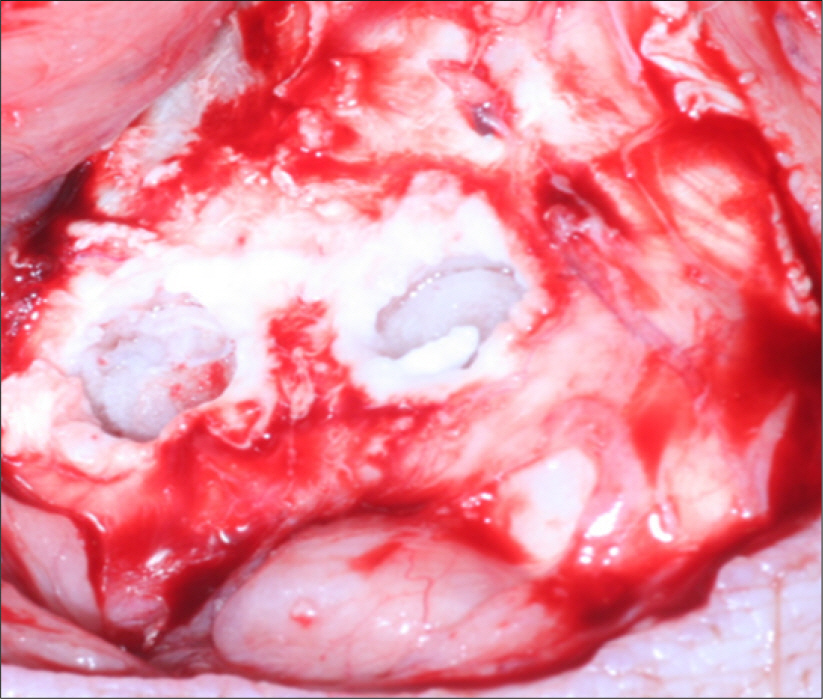
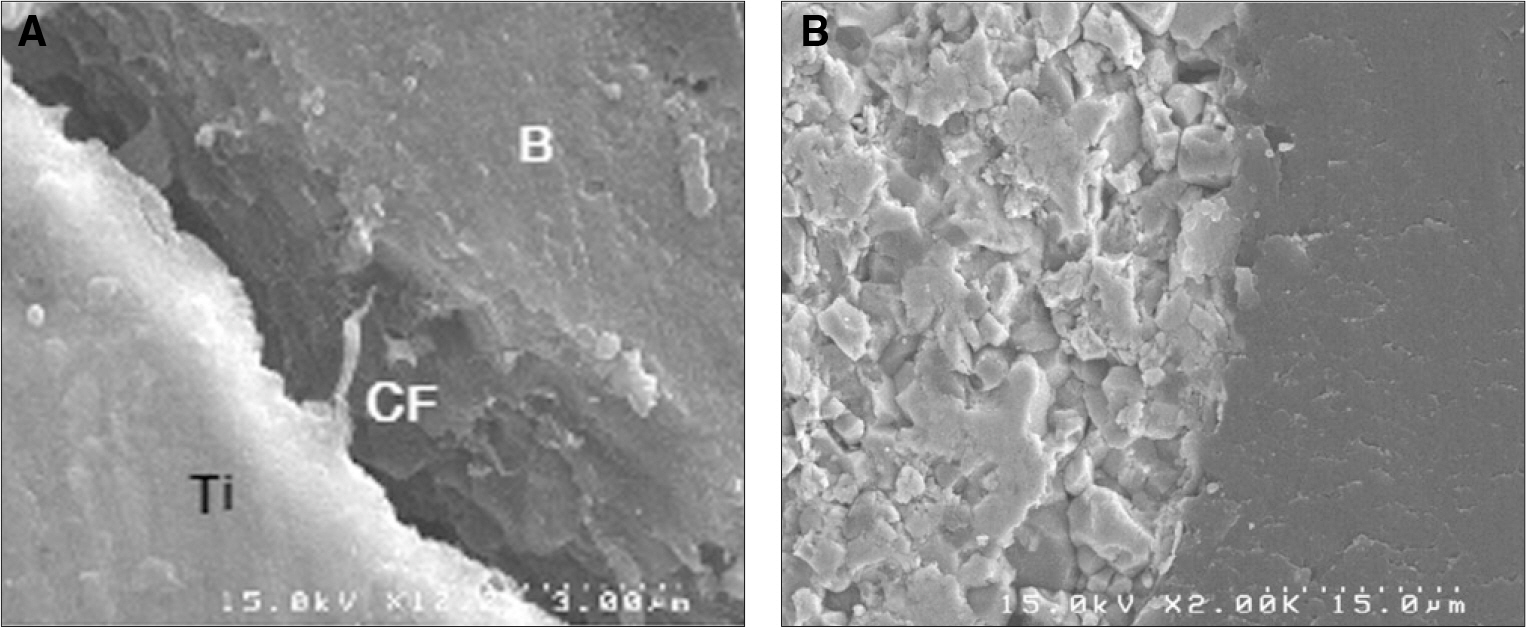
Specimens were divided into two groups; the control group was experimented with eighteen titanium implants which had anodized surface and the experimental group was experimented with eighteen sandblasted zirconia (Y-TZP) implants. At the tibias of six pigs, implants were installed into bone defect sites prepared surgically and treated with resorbable membranes and bovine bone. Two pigs were sacrificed after 1, 4 and 12 weeks respectively. Each implant site was sampled and processed for histologic and histomorphometric analysis. The stability of implants was evaluated with a Periotes(R). And the interfaces between bone and the implant were observed with a scanning electron microscope.
RESULTS
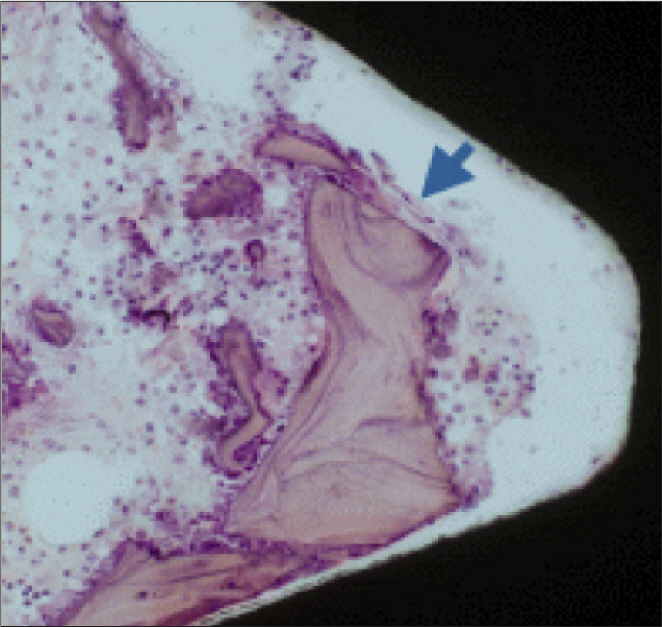
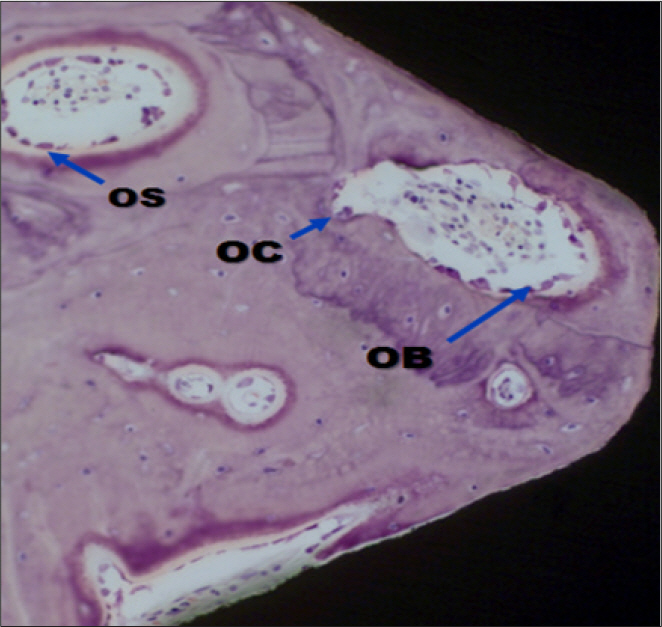
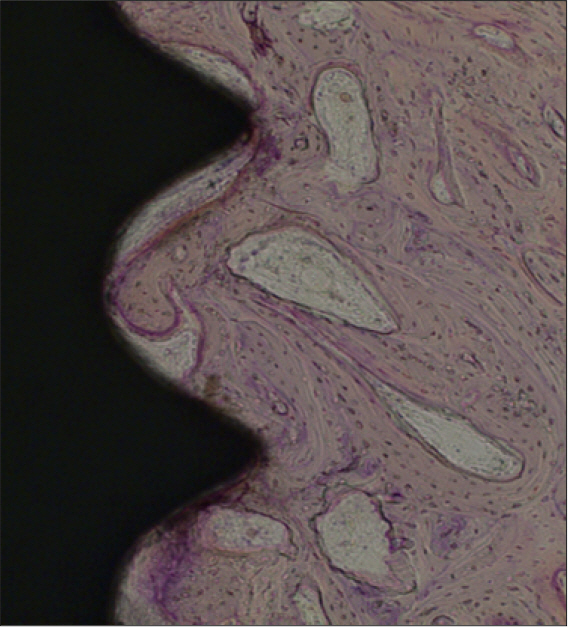
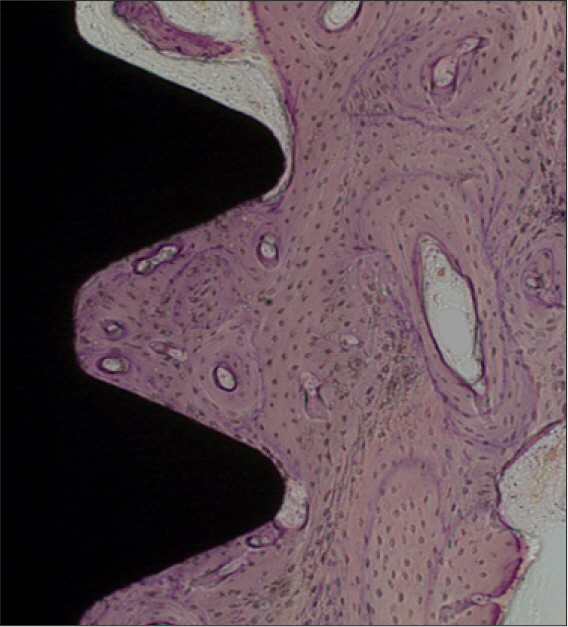
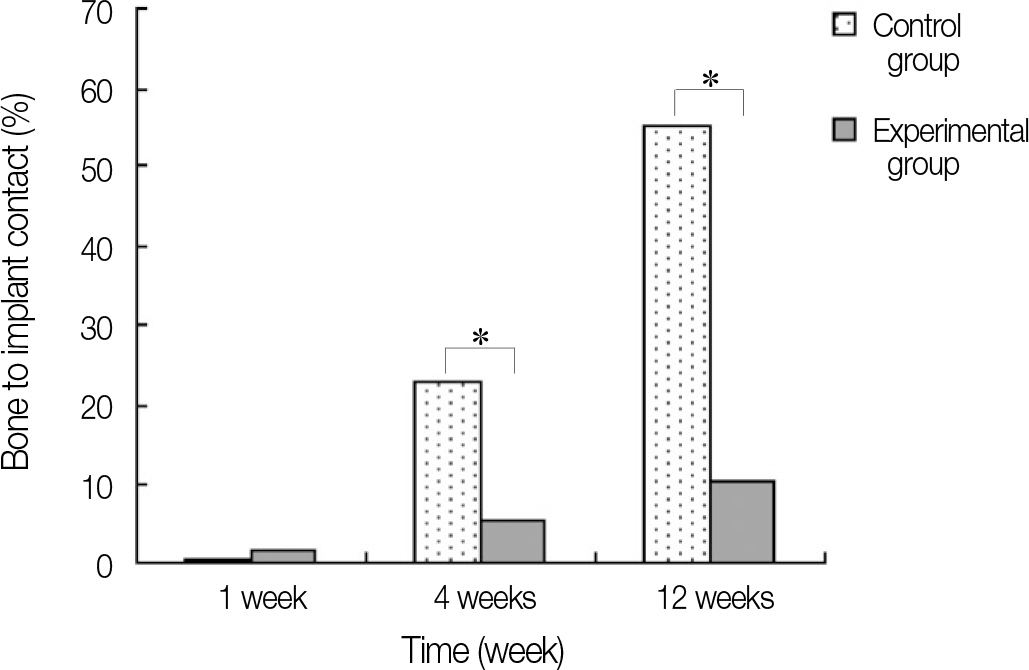
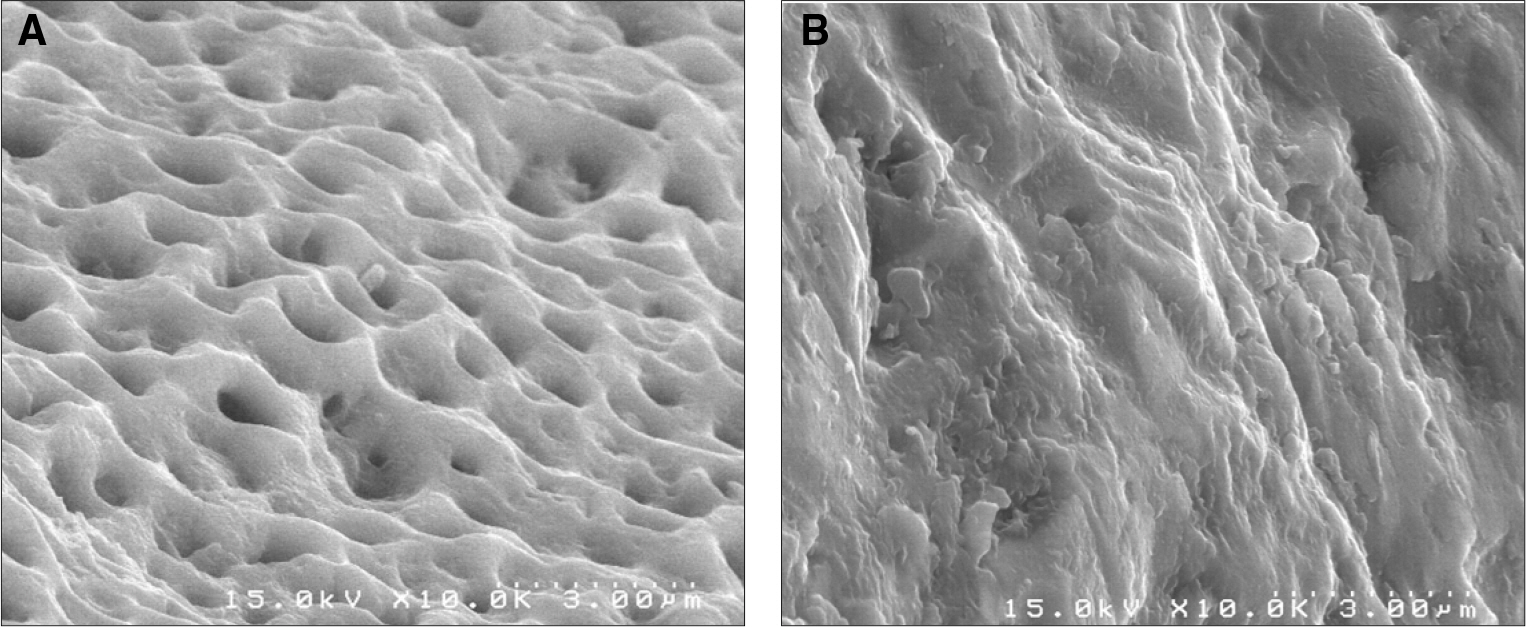
In stability analysis there was no significant difference between Periotest values of the control group and the experimental group. In histologic analysis with a light microscope after 4 weeks, there was new bone formation with the resorption of bovine bone and the active synthesis of osteoblasts in both groups. In bone-implant contact percentage there was significant difference between both groups (P<.05). In bone area percentage there was no significant difference between both groups. In analysis of both groups with a scanning electron microscope there was a gap between bone and a surface at 4 weeks and it was filled up with bone formed newly at 12 weeks.
CONCLUSION
When accompanied by xenograft using membrane, bone to implant contact percentage of zirconia implants used in this experiment was significantly less than that of the titanium implants by surface treatment of anodic oxidation. So, it is considered that the improvement of zirconia implant is needed through ongoing research on surface treatment methods for its practical use.
MeSH Terms
Figure
Reference
-
1.Piconi C., Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999. 20:1–25.
Article2.Scarano A., Di Carlo F., Quaranta M., Piattelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003. 29:8–12.
Article3.Kohal RJ., Weng D., Ba¨chle M., Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004. 75:1262–8.
Article4.Song YS., Cho IH. Surface characteristics and stability of implants treated with alkali and heat. J Korean Acad Prosthodont. 2008. 46:490–9.
Article5.Langhoff JD., Voelter K., Scharnweber D., Schnabelrauch M., Schlottig F., Hefti T., Kalchofner K., Nuss K., von Rechenberg B. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: a study in sheep. Int J Oral Maxillofac Surg. 2008. 37:1125–32.
Article6.Nyman S. Bone regeneration using the principle of guided tissue regeneration. J Clin Periodontol. 1991. 18:494–8.
Article7.Ha¨mmerle CH., Olah AJ., Schmid J., Flu¨ckiger L., Gogolewski S., Winkler JR., Lang NP. The biological effect of natural bone mineral on bone neoformation on the rabbit skull. Clin Oral Implants Res. 1997. 8:198–207.8.Lim JH., Lim JH., Lim HS., Cho IH. The effect of various graft materials on the stability of implant and peri-implant tissue response in rabbit tibia. J Korean Acad Oral Maxillofac Implantol. 2001. 5:41–64.9.Ha¨mmerle CH., Jung RE., Yaman D., Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008. 19:19–25.
Article10.Park C., Lim JH., Cho IH., Lim HS. A study on the measurement of the implant stability using resonance frequency analysis. J Korean Acad Prosthodont. 2003. 41:182–206.11.Neugebauer J., Iezzi G., Perrotti V., Fischer JH., Khoury F., Piattelli A., Zoeller JE. Experimental immediate loading of dental implants in conjunction with grafting procedures. J Biomed Mater Res B Appl Biomater. 2009. 91:604–12.
Article12.Wang S., Liu Y., Fang D., Shi S. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007. 13:530–7.
Article13.Jang ES., Park JW., Kweon H., Lee KG., Kang SW., Baek DH., Choi JY., Kim SG. Restoration of peri-implant defects in immediate implant installations by Choukroun platelet-rich fibrin and silk fibroin powder combination graft. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010. 109:831–6.
Article14.Akimoto K., Becker W., Persson R., Baker DA., Rohrer MD., O'Neal RB. Evaluation of titanium implants placed into simulated extraction sockets: a study in dogs. Int J Oral Maxillofac Implants. 1999. 14:351–60.15.Schultze-Mosgau S., Schliephake H., Radespiel-Tro¨ger M., Neukam FW. Osseointegration of endodontic endosseous cones: zirconium oxide vs titanium. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000. 89:91–8.16.Lee SH., Cho IH. Surface characteristics and bioactivity of anodically oxidized titanium surfaces. J Korean Acad Prosthodont. 2007. 45:85–97.17.Gahlert M., Gudehus T., Eichhorn S., Steinhauser E., Kniha H., Erhardt W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin Oral Implants Res. 2007. 18:662–8.
Article18.Schliephake H., Hefti T., Schlottig F., Ge′det P., Staedt H. Mechanical anchorage and peri-implant bone formation of surface-modified zirconia in minipigs. J Clin Periodontol. 2010. 37:818–28.
Article19.Yamashita D., Machigashira M., Miyamoto M., Takeuchi H., Noguchi K., Izumi Y., Ban S. Effect of surface roughness on initial responses of osteoblast-like cells on two types of zirconia. Dent Mater J. 2009. 28:461–70.
Article20.Lincks J., Boyan BD., Blanchard CR., Lohmann CH., Liu Y., Cochran DL., Dean DD., Schwartz Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998. 19:2219–32.
Article21.Nishimoto SK., Nishimoto M., Park SW., Lee KM., Kim HS., Koh JT., Ong JL., Liu Y., Yang Y. The effect of titanium surface roughening on protein absorption, cell attachment, and cell spreading. Int J Oral Maxillofac Implants. 2008. 23:675–80.22.Rocchietta I., Fontana F., Addis A., Schupbach P., Simion M. Surface-modified zirconia implants: tissue response in rabbits. Clin Oral Implants Res. 2009. 20:844–50.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osseointegration of zirconia implant in the tibia of pigs
- Comparative Evaluation of Osseointegration Parameters between Titanium and Zirconia Implants Placed in Beagle Dogs
- Tissue integration of zirconia and titanium implants with and without buccal dehiscence defects
- Comparison of marginal and internal fit of zirconia abutments with titanium abutments in internal hexagonal implants
- Effects of counter torque and transposition (transfer) of installed implants timing on their integration in dog tibia