Korean J Urol.
2007 Mar;48(3):244-251. 10.4111/kju.2007.48.3.244.
The Relationship of Clusterin Expression with Ki-67 Expression and Clinicopathological Factors in Human Renal Cell Carcinoma
- Affiliations
-
- 1From the Department of Urology, College of Medicine, Pusan National University, Busan, Korea. mkchung@pusan.ac.kr
- KMID: 1997120
- DOI: http://doi.org/10.4111/kju.2007.48.3.244
Abstract
- PURPOSE
This study was performed to evaluate the relationship of the expressions of clusterin with Ki-67 and the clinicopathological factors and significance of clusterin expression in human renal cell carcinoma (RCC).
MATERIALS AND METHODS
Normal kidney (n=26) and RCC tissues (n=111) were obtained from 111 patients who had undergone a radical or partial nephrectomy. The expressions of clusterin and Ki-67 protein were analysed using immunohistochemical staining. The medical records of the patients were retrospectively reviewed to evaluate the clinicopathological factors.
RESULTS
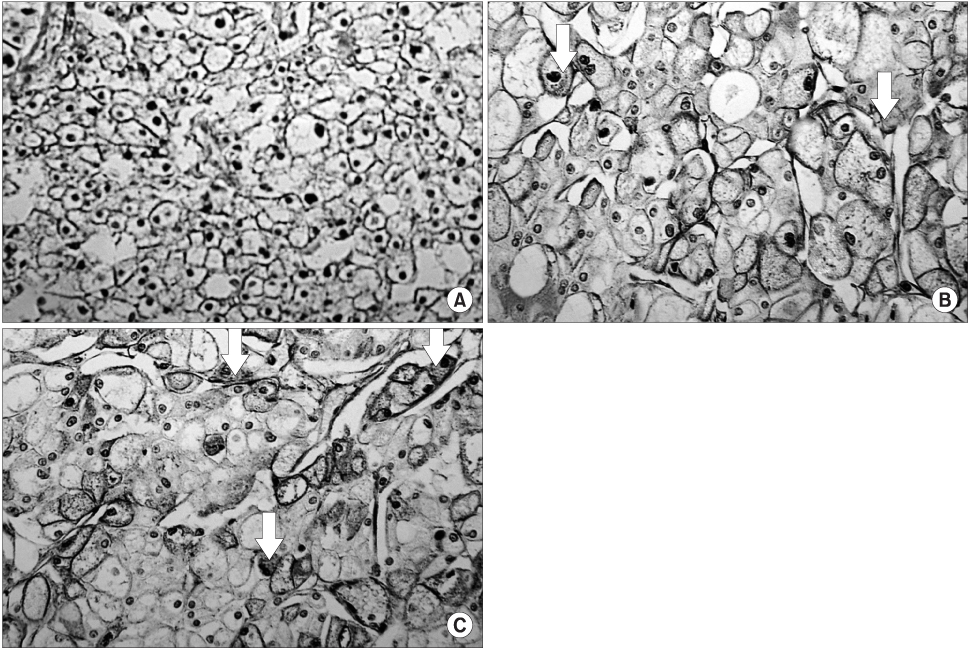
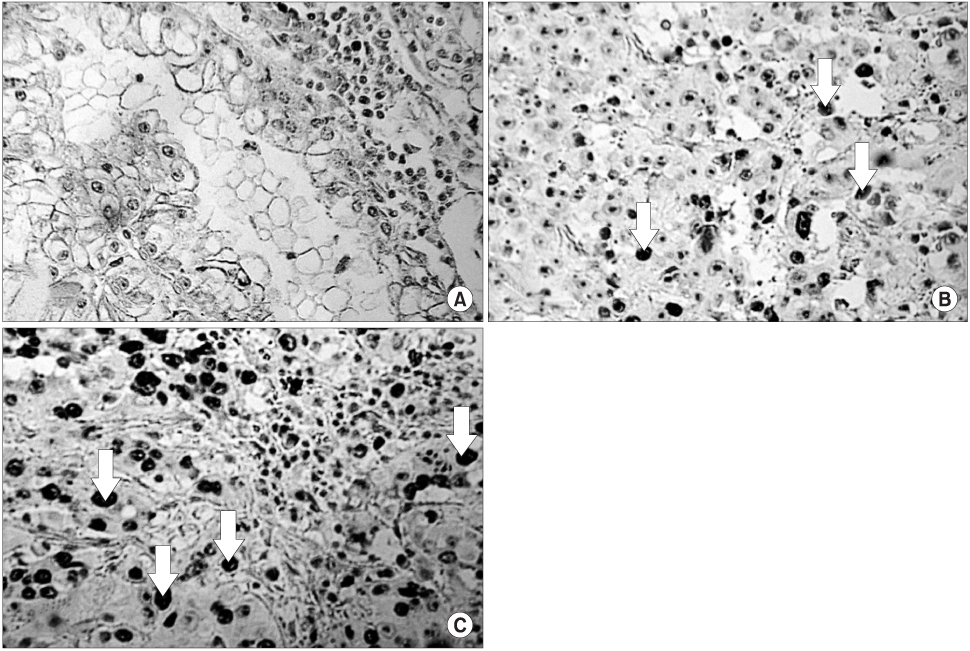
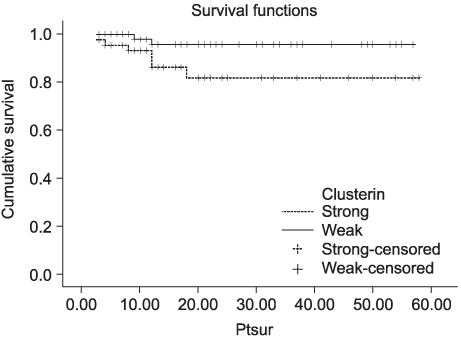
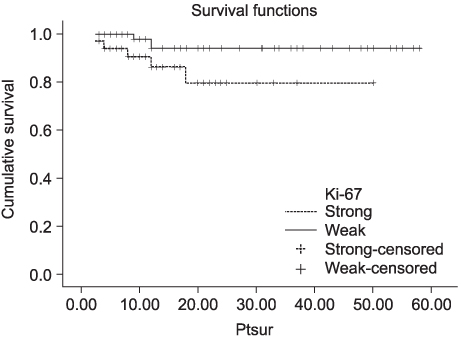
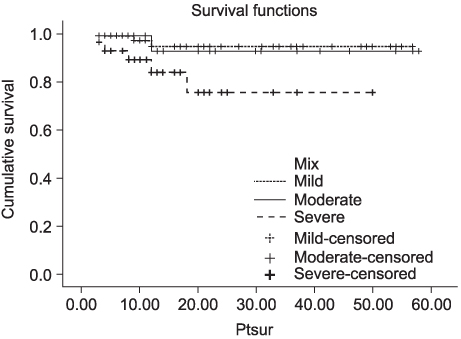
In contrast to the clusterin and Ki-67 expressions of 30.8 and 11.5%, respectively in the normal kidney (n=26), those in the RCC tissues were 93.7 and 28.8% (n=111), respectively (p<0.05). In contrast to the normal tissues, Ki-67 staining was significantly correlated with the expression of clusterin in the RCC tissues (p<0.05). The expression level of clusterin protein in the RCC tissues was significantly related to the tumor stage and grade (p<0.05), but not to age, gender or histological cell type (p>0.05). Furthermore, the recurrence-free survival in patients with strong clusterin expression was significantly lower than in those with weak expression (p<0.05).
CONCLUSIONS
These findings suggest that clusterin may be involved in the progression of RCC and; therefore, a useful prognostic variable in patients with an RCC.
MeSH Terms
Figure
Cited by 1 articles
-
The Relationship of Clusterin Expression and Ki-67 Labeling Index with Clinicopathologic Factors in Human Transitional Cell Carcinoma
Won Hee Chon, Sang Don Lee, Jeong Zoo Lee, Kyung Woon Choi
Korean J Urol. 2008;49(8):688-695. doi: 10.4111/kju.2008.49.8.688.
Reference
-
1. Weiss RH, Lin PY. Kidney cancer: identification of novel targets for therapy. Kidney Int. 2006. 69:224–232.2. Motzer RJ, Bander NH, Nanus DM. Renal cell carcinoma. N Engl J Med. 1996. 335:865–875.3. Sylvester SR, Morales C, Oko R, Griswold MD. Localization of sulfated glycoprotein-2 (clusterin) on spermatozoa and in the reproductive tract of the male rat. Biol Reprod. 1991. 45:195–207.4. Parczyk K, Pilarsky C, Rachel U, Koch-Brandt C. Gp 80 (clusterin; TRPM-2) mRNA level is enhanced in human renal clear cell carcinoma. J Cancer Res Clin Oncol. 1994. 120:186–188.5. Slawin K, Sawczuk IS, Olsson CA, Buttyan R. Chromosomal assignment of the human homologue encoding SGP-2. Biochem Biophys Res Commun. 1990. 172:160–164.6. Chevalier RL, Chung KH, Smith CD, Ficenec M, Gomez RA. Renal apoptosis and clusterin following ureteral obstruction: the role of maturation. J Urol. 1996. 156:1474–1479.7. Kelin T, Miller MI, Buttyan R, Raffo AJ, Burchard M, Devris G. Apoptosis in the rat penis after penile denervation. J Urol. 1997. 158:626–630.8. Rosenberg ME, Silkensen J. Clusterin and the kidney. Exp Nephrol. 1995. 3:9–14.9. Mellon K, Neal DE, Robinson MC, Marsh C, Wright C. Cell cycling in bladder carcinoma determined by monoclonal antibody Ki 67. Br J Urol. 1990. 66:281–285.10. Okamura K, Miyake K, Koshikawa T, Asai J. Growth fractions of transitional cell carcinomas of the bladder defined by the monoclonal antibody Ki-67. J Urol. 1990. 144:875–878.11. Lakin J, Bennett SA, Chen JH, Arnold JM, Morrissey C, Wong P. Clusterin biogenesis in altered during apoptosis in the regressing rat ventral prostate. J Biol Chem. 1998. 273:27887–27895.12. Ahuja HS, Tenniswood M, Lockshin R, Zakrei ZF. Expression of clusterin in cell differentiation and cell death. Biochem Cell Biol. 1994. 72:523–530.13. Rittmaster RS, Magor KE, Manning AP, Norman RW, Lazier DB. Differential effect of 5 alpha-reductase inhibition and castration on androgen-regulated gene expression in rat prostate. Mol Endocrinol. 1991. 5:1023–1029.14. Nickerson T, Pollak M, Huynh H. Castration-induced apoptosis in the rat ventral prostate is associated with increased expression of gene encoding insulin-like growth factor binding protein 2, 3, 4 and 5. Endocrinology. 1998. 139:807–810.15. Yamada K, Hori Y, Hanafusa N, Okuda T, Nagano N, Choi-Miura NH, et al. Clusterin is up-regulated in glomerular mesangial cells in complement-mediated injury. Kidney Int. 2001. 59:137–146.16. Schwochau GB, Nath KA, Rosenberg ME. Clusterin protects against oxidative stress in vitro through aggregative and nonaggregative properties. Kidney Int. 1998. 53:1647–1653.17. Dvergsten J, Manivel JC, Correa-Rotter R, Rosenberg ME. Expression of clusterin in human renal diseases. Kidney Int. 1994. 45:828–835.18. Miyake H, Hara S, Arakawa S, Kamidono S, Hara I. Over expression of clusterin is an independent prognostic factor for nonpapillary renal cell carcinoma. J Urol. 2002. 167:703–706.19. Bubendorf L, Tapia C, Gasser TC, Casella R, Grunder B, Moch H. Ki-67 labeling index in core needle biopsies independently predicts tumor-specific survival in prostate cancer. Hum Pathol. 1998. 29:949–954.20. Cohen MB, Waldman FM, Carroll PR, Kerschmann R, Chew K, Mayall BH. Comparison of five histopathologic methods to assess cellular proliferation in transitional cell carcinoma of the urinary bladder. Hum Pathol. 1993. 24:772–778.21. Sengupta S, Lohse CM, Leibovich BC, Frank I, Thompson RH, Webster WS. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005. 104:511–520.22. Delahunt B, Bethwaite PB, Thornton A, Ribas JL. Proliferation of renal cell carcinoma assessed by fixation-resistant polyclonal Ki-67 labeling. Cancer. 1995. 75:2714–2719.23. Rioux-Leclercq N, Turlin B, Bansard J, Patard J, Manunta A, Moulinoux JP. Value of immunohistochemical Ki-67 and p53 determinations as predictive factors of outcome in renal cell carcinoma. Urology. 2000. 55:501–505.24. Visapaa H, Bui M, Huang Y, Seligson D, Tsai H, Pantuck A. Correlation of Ki-67 and gelsolin expression to clinical outcome in renal clear cell carcinoma. Urology. 2003. 61:845–850.25. Kurahashi T, Muramaki M, Yamanaka K, Hara I, Miyake H. Expression of the secreted form of clusterin protein in renal cell carcinoma as a predictor of disease extension. BJU Int. 2005. 96:895–899.26. Kadomatsu K, Anzano MA, Slayter MV, Winokur TS, Smith JM, Sporn MB. Expression of sulfated glycoprotein 2 is associated with carcinogenesis induced by N-nitroso-N-methylurea in rat prostate and seminal vesicle. Cancer Res. 1993. 53:1480–1483.27. Steinberg J, Oyasu R, Lang S, Sintich S, Rademaker A, Lee C. Intracellular levels of SGP-2 (clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res. 1997. 3:1707–1711.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship of Clusterin Expression and Ki-67 Labeling Index with Clinicopathologic Factors in Human Transitional Cell Carcinoma
- Reduced Expression of E-cadherin in Human Non-small Cell Lung Carcinoma
- Caveolin-1 and Ki-67 Expression as Prognostic Factors in Clear Cell Carcinoma of the Kidney
- The expression of clusterin, bax, p53, Ki-67, and apoptotic index in epithelial ovarian tumors
- Correlation of Clinical Stage and Presumptive Prognostic Factors in Renal Cell Carcinoma