Korean J Urol.
2007 Jul;48(7):684-690. 10.4111/kju.2007.48.7.684.
Estramustine Phosphate Based Chemotherapy for Hormone Refractory Prostate Cancer
- Affiliations
-
- 1From the Department of Urology, Urologcial Science Institute, Yonsei University College of Medicine, Seoul, Korea. sjhong346@yumc.yonsei.ac.kr
- KMID: 1990218
- DOI: http://doi.org/10.4111/kju.2007.48.7.684
Abstract
-
PURPOSE: We wanted to evaluate the efficacy and side effects of estramustine monotherapy and estramustine plus etoposide or dexamethasone combined therapies for patients with hormone refractory prostate cancer(HRPC).
MATERIALS AND METHODS
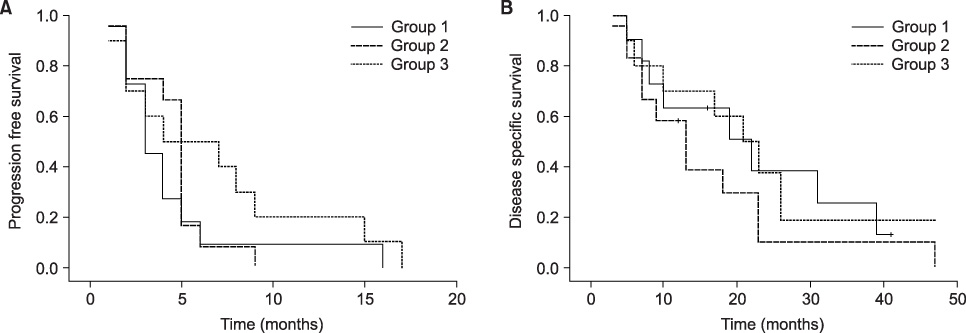
Between 2000 and 2004, 33 patients who were diagnosed with HRPC and treated with estramustine-based chemotherapy were evaluated. Eleven patients had oral estramustine monotherapy(group 1), 12 patients had oral estramustine plus oral etoposide(group 2), and finally 10 patients had oral estramustine plus oral dexamethasone(group 3). The prostate-specific antigen(PSA) response, progression-free survival and disease-specific survival were evaluated.
RESULTS
The median patient age was 71 years and the median PSA was 97.3ng/ml. The median follow-up period was 17 months(range: 5-47). The overall response rate was 45.5%, and the response rate for each group was 36.4% for group 1, 41.7% for group 2 and 70.0% for group 3, respectively. The median time to progression(TTP) was 5 months(range: 1-16) overall and it was 5 months, 5.5 months and 5 months in groups 1, 2 and 3, respectively. Regarding the response rate, progression-free survival and disease specific survival, there were no statistically significant differences between the three groups(p>0.05). The most common hematologic complication was anemia that occurred in 28 patients and deep vein thrombosis occurred in 2. Severe toxicities(>or=grade 3) occurred in only 2 patients.
CONCLUSIONS
Estramustine phosphate showed over a 45% response rates with less morbidities. Estramustine-based chemotherapy can be considered as an option for the treatment of HRPC. However, larger randomized controlled trials for regimens combined with other efficacious agents are necessary to elucidate the efficacy of chemotherapy for HRPC.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004. 101:3–27.2. Ministry of Health and Welfare. 2004 Annual Report of the Central Cancer Registry in Korea. 2005.3. Shin JS, Choi HJ, Choi YS, Chai SE, Choi HY. Predictive factor for the early progression of androgen independent prostate cancer in intermittent androgen deprivation therapy. Korean J Urol. 2004. 45:858–864.4. Petrylak DP, Tangen CM, Hussain HA, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004. 351:1513–1520.5. Hudes G, Greenberg R, Krigel RL, Fox S, Scher R, Litwin S, et al. Phase-II study of estramustine and vinblastine, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol. 1992. 10:1754–1761.6. Hartley-Asp B, Kruse E. Nuclear protein matrix as a target for estramustine-induced cell death. Prostate. 1986. 9:387–395.7. Yagoda A, Smith JA Jr, Soloway MS. Phase II study of estramustine phosphate in advenced hormone refractory prostate cancer with increasing prostate specific antigen levels. J Urol. 1991. 145:Suppl. 384.8. Iversen P, Rasmussen F, Asmussen C, Christensen IJ, Eickhoff J, Klarskov P, et al. Danish Prostatic Cancer Group. Estramustine phosphate versus placebo as second line treatment after orchiectomy in patients with metastatic prostate cancer: DAPROCA study 9002. J Urol. 1997. 157:929–934.9. Winquist E, Waldron T, Berry S, Ernst DS, Hotte S, Lukka H. Non-hormonal systemic therapy in men with hormone-refractory prostate cancer and metastases: a systematic review from the Cancer Care Ontario Program in Evidence-based Care's Genitourinary Cancer Disease Site Group. BMC Cancer. 2006. 6:112.10. Miller AB, Hoogstraten B, Staquet M, Winker A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.11. Pienta K, Redman B, Hussain M, Cummings G, Esper PS, Appel C, et al. Phase-II study of estramustine and oral etoposide in hormone-refractory adenocarcinoma of the prostate. J Clin Oncol. 1994. 12:2005–2012.12. Berruti A, Fara E, Tucci M, Tarabuzzi R, Mosca A, Terrone C, et al. Oral estramustine plus oral etoposide in the treatment of hormone refractory prostate cancer patients: a phase II study with a 5-year follow-up. Urol Oncol. 2005. 23:1–7.13. Hudes G, Einhorn L, Ross E, Balsham A, Loehrer P, Ramsey H, et al. Vinblastine versus vinblastine plus oral estramustine phosphate for patients with hormone-refractory prostate cancer: A Hoosier Oncology Group and Fox Chase Network phase III trial. J Clin Oncol. 1999. 17:3160–3166.14. Hudes G, Ross E, Roth B. Improved survival for patients with hormone-refractory prostate cancer receiving estramustine-based antimicrotubule therapy: final report of a Hoosier oncology group and Fox Chase network phase III trial comparing vinblastine and vinblastine plus estramustine phosphate. Proc Am Soc Clin Oncol. 2002. 21:Suppl. 177. abstract 704.15. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004. 351:1502–1512.16. Hussain MH, Pienta KJ, Redman BG, Cummings GD, Flaherty LE. Oral etoposide in the treatment of hormone-refractory prostate cancer. Cancer. 1994. 74:100–103.17. Armstrong AJ, Carducci MA. Kirby RS, Partin AW, Feneley M, Parsons JK, editors. Chemotherapy for advanced prostate cancer. Prostate cancer. Principles and practice. 2006. 1st ed. London: Taylor&Francis;989–1003.18. Nishimura K, Nonomura N, Satoh E, Harada Y, Nakayama M, Tokizane T, et al. Potential mechanism for the effects of dexamethasone on growth of androgen-indepenent prostate cancer. J Natl Cancer Inst. 2001. 93:1739–1746.19. Johansson JE, Andersson SO, Holmberg L. High-dose medroxyprogesterone acetate versus estramustine in therapy-resistant prostatic cancer: a randomised study. Br J Urol. 1991. 68:67–73.20. de Kernion JN, Murphy GP, Priore R. Comparison of flutamide and Emcyt in hormone-refractory metastatic prostatic cancer. Urology. 1988. 31:312–317.21. Tay MH, Nakabayashi M, Oh WK. Vogelzang NJ, Scardino PT, Shipley WU, Debruyne FM, Linehan WM, editors. Management of hormone refractory prostate cancer. Comprehensive textbook of genitourinary oncology. 2006. 3rd ed. Philadelphia: Williams&Wilkins;341–352.22. Lubiniecki GM, Berlin JA, Weistein RB, Vaughn DJ. Thromboembolic events with estramustine phosphate-based chemotherapy in patients with hormone-refractory prostate carcinoma: results of a meta-analysis. Cancer. 2004. 101:2755–2759.23. Sinibaldi VJ, Carducci MA, Moore-Cooper S, Laufer M, Zahurak M, Eisenberger MA, et al. Phase II evaluation of docetaxel plus one-day oral estramustine phosphate in the treatment of patients with androgen independent prostate carcinoma. Cancer. 2002. 94:1457–1465.24. Oh WK, Halabi S, Kelly WK, Werner C, Godley PA, Vogelzang NJ, et al. A phase II study of estramustine, docetaxel, and carboplatin with granulocyte-colony-stimulating factor support in patients with hormone-refractory prostate carcinoma: Cancer and Leukemia Group B 99813. Cancer. 2003. 98:2592–2598.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dramatic Decline of PSA and Symptom Improvement after Estramustine Withdrawal in a Hormone-refractory Prostate Cancer Patient
- Estramustine Phosphate Monotherapy in Castration-Resistant Prostate Cancer Patients Who Cannot Receive Cytotoxic Chemotherapy
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Comparison of Ketoconazole and Estramustine for Treating Patients with Castration-Resistant Prostate Cancer
- What's New in Hormone-refractory Prostate Cancer Treatment