J Adv Prosthodont.
2014 Aug;6(4):285-294. 10.4047/jap.2014.6.4.285.
Characteristics and osteogenic effect of zirconia porous scaffold coated with beta-TCP/HA
- Affiliations
-
- 1Department of Prosthodontics, College of Dentistry, Dankook University, Cheonan, Republic of Korea. cho8511@dankook.ac.kr
- KMID: 1974848
- DOI: http://doi.org/10.4047/jap.2014.6.4.285
Abstract
- PURPOSE
The purpose of this study was to evaluate the properties of a porous zirconia scaffold coated with bioactive materials and compare the in vitro cellular behavior of MC3T3-E1 preosteoblastic cells to titanium and zirconia disks and porous zirconia scaffolds.
MATERIALS AND METHODS
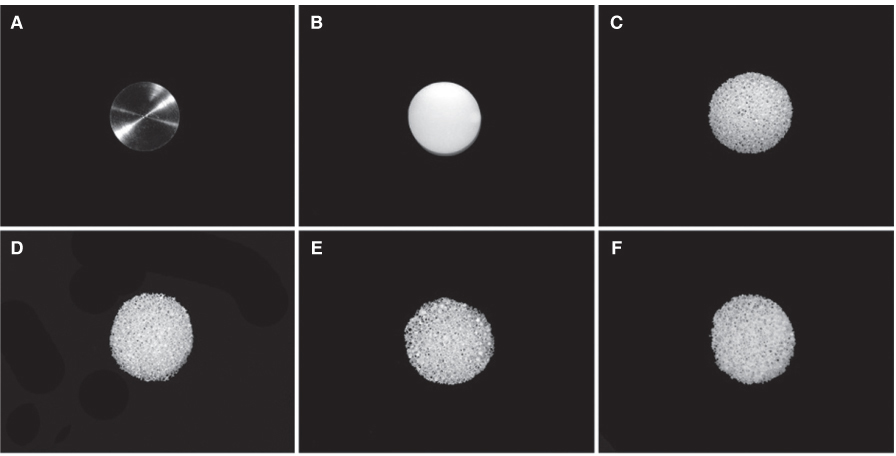
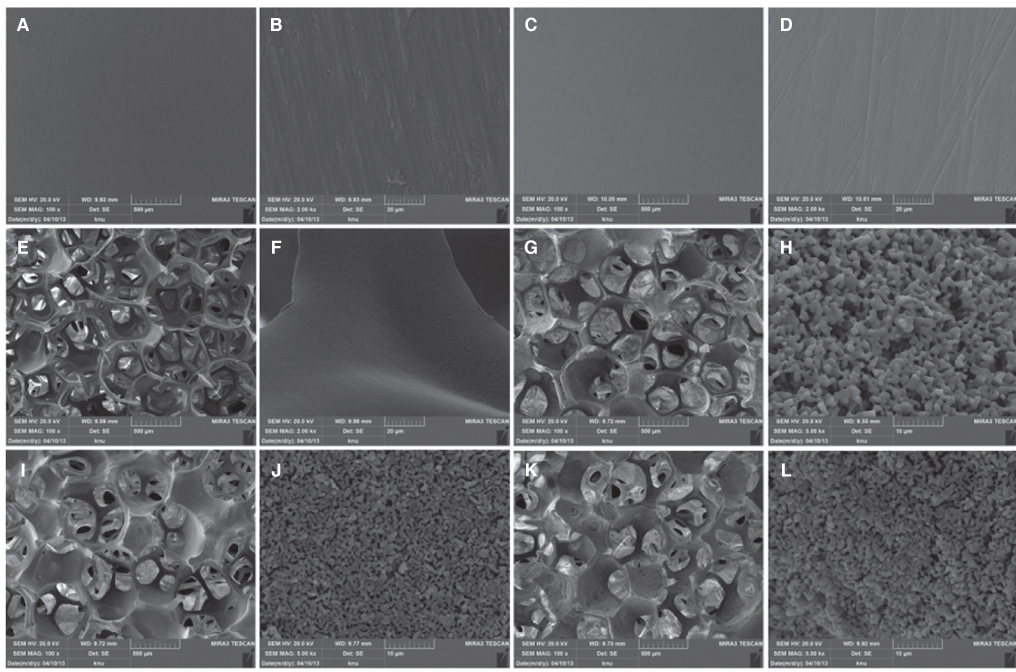
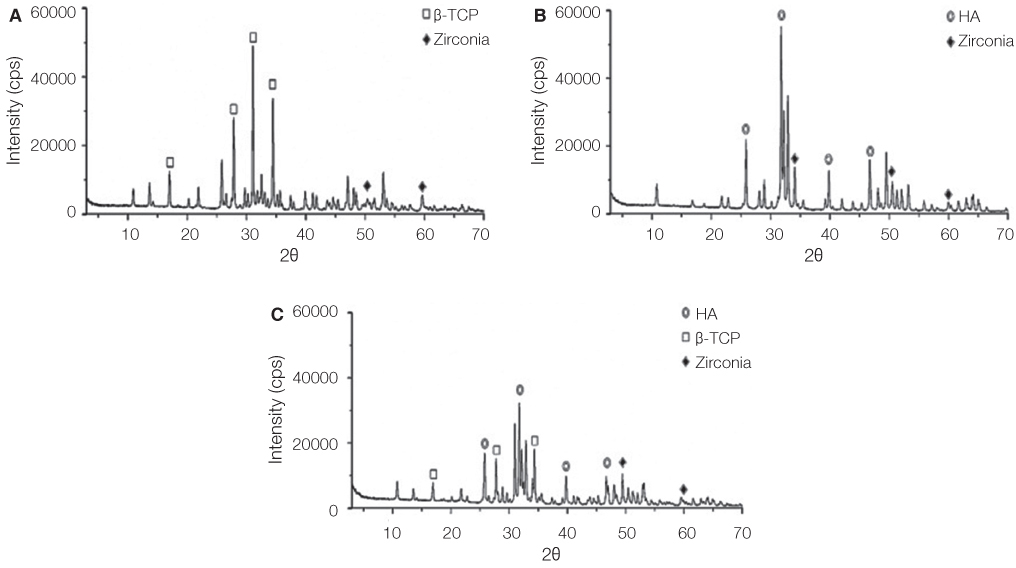
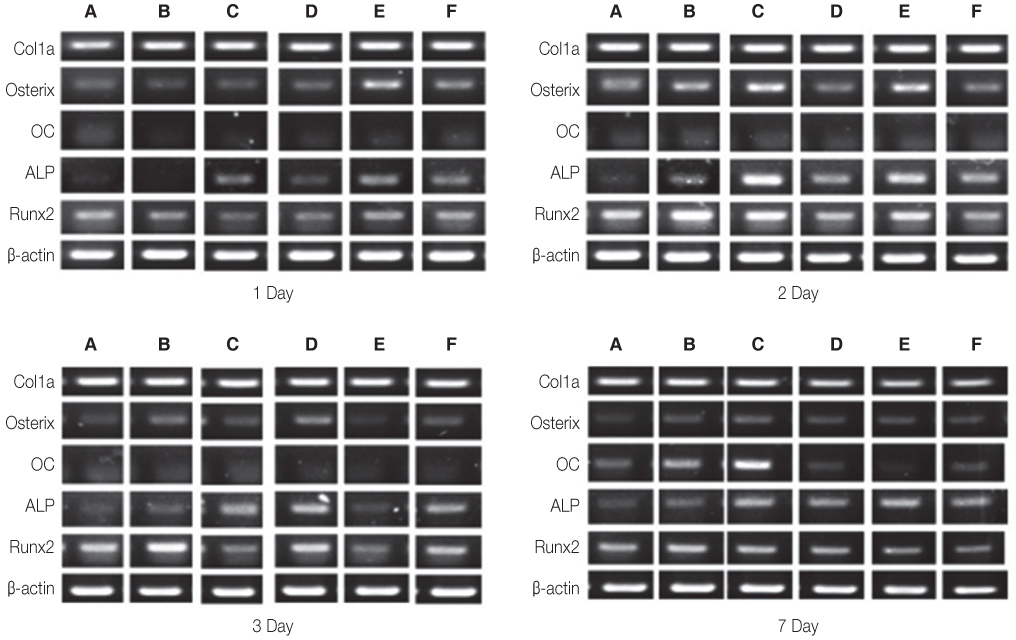
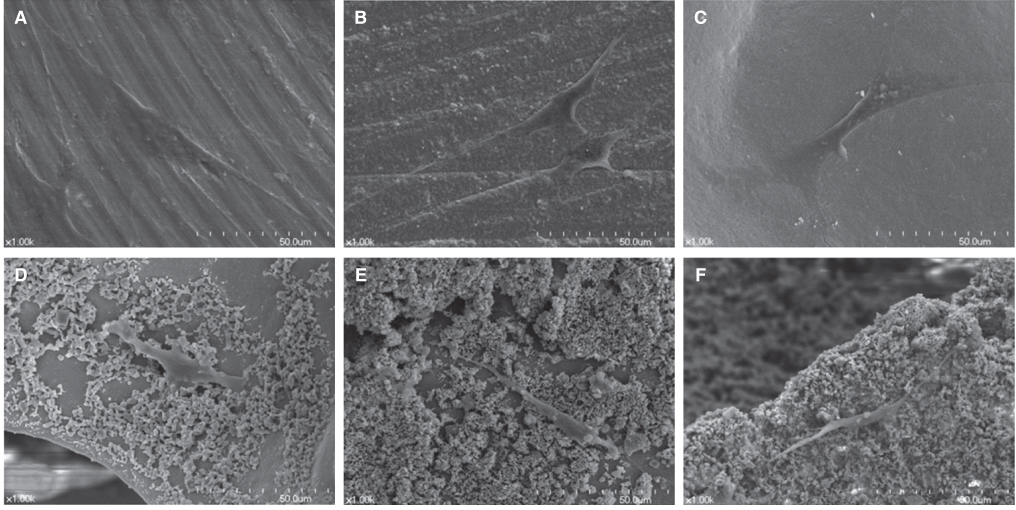
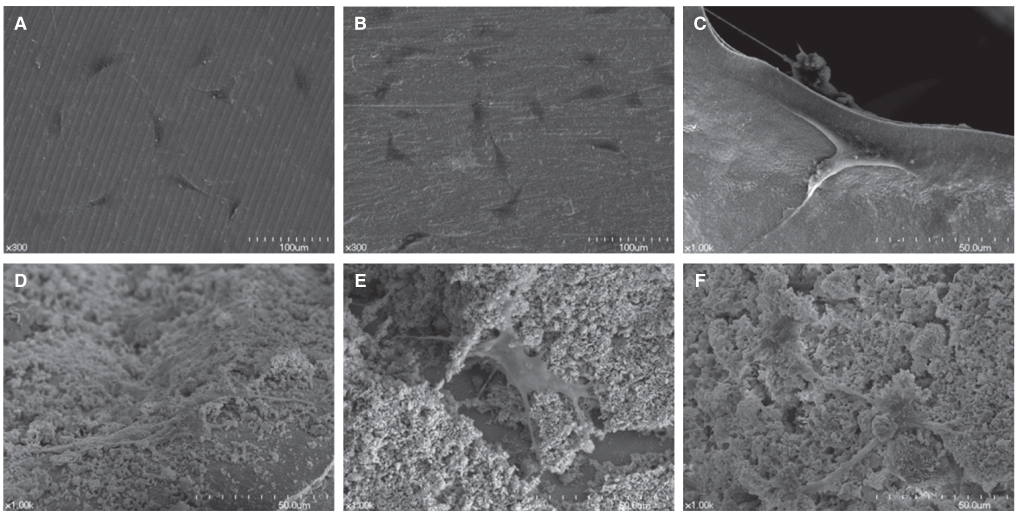
Titanium and zirconia disks were prepared. A porous zirconia scaffold was fabricated with an open cell polyurethane disk foam template. The porous zirconia scaffolds were coated with beta-TCP, HA and a compound of beta-TCP and HA (BCP). The characteristics of the specimens were evaluated using scanning electron microscopy (SEM), energy dispersive x-ray spectrometer (EDX), and x-ray diffractometry (XRD). The dissolution tests were analyzed by an inductively coupled plasma spectrometer (ICP). The osteogenic effect of MC3T3-E1 cells was assessed via cell counting and reverse transcriptase-polymerase chain reaction (RT-PCR).
RESULTS
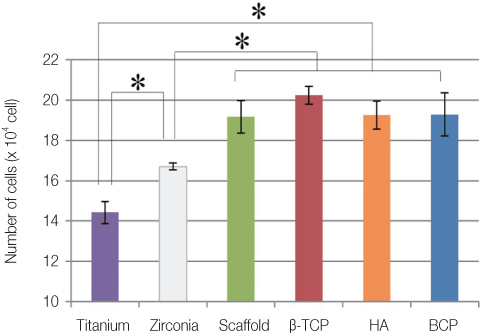
The EDX profiles showed the substrate of zirconia, which was surrounded by the Ca-P layer. In the dissolution test, dissolved Ca2+ ions were observed in the following decreasing order; beta-TCP > BCP > HA (P<.05). In the cellular experiments, the cell proliferation on titanium disks appeared significantly lower in comparison to the other groups after 5 days (P<.05). The zirconia scaffolds had greater values than the zirconia disks (P<.05). The mRNA level of osteocalcin was highest on the non-coated zirconia scaffolds after 7 days.
CONCLUSION
Zirconia had greater osteoblast cell activity than titanium. The interconnecting pores of the zirconia scaffolds showed enhanced proliferation and cell differentiation. The activity of osteoblast was more affected by microstructure than by coating materials.
MeSH Terms
Figure
Cited by 1 articles
-
Comparative study of new bone formation capability of zirconia bone graft material in rabbit calvarial
Ik-Jung Kim, Soo-Yeon Shin
J Adv Prosthodont. 2018;10(3):167-176. doi: 10.4047/jap.2018.10.3.167.
Reference
-
1. Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002; 295:1014–1017.2. Fransson C, Lekholm U, Jemt T, Berglundh T. Prevalence of subjects with progressive bone loss at implants. Clin Oral Implants Res. 2005; 16:440–446.3. Klinge B. Peri-implant marginal bone loss: an academic controversy or a clinical challenge? Eur J Oral Implantol. 2012; 5:S13–S19.4. Kim YK, Kim SG, Yun PY, Yeo IS, Jin SC, Oh JS, Kim HJ, Yu SK, Lee SY, Kim JS, Um IW, Jeong MA, Kim GW. Autogenous teeth used for bone grafting: a comparison with traditional grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 117:e39–e45.5. Bae JH, Kim YK, Kim SG, Yun PY, Kim JS. Sinus bone graft using new alloplastic bone graft material (Osteon)-II: clinical evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:e14–e20.6. Dalkýz M, Ozcan A, Yapar M, Gökay N, Yüncü M. Evaluation of the effects of different biomaterials on bone defects. Implant Dent. 2000; 9:226–235.7. Carotenuto G, Spagnuolo G, Ambrosio L, Nicolais L. Macroporous hydroxyapatite as alloplastic material for dental applications. J Mater Sci Mater Med. 1999; 10:671–676.8. Lu J, Descamps M, Dejou J, Koubi G, Hardouin P, Lemaitre J, Proust JP. The biodegradation mechanism of calcium phosphate biomaterials in bone. J Biomed Mater Res. 2002; 63:408–412.9. Chao SY, Poon CK. Histologic study of tissue response to implanted hydroxylapatite in two patients. J Oral Maxillofac Surg. 1987; 45:359–362.10. Frame JW, Browne RM, Brady CL. Hydroxyapatite as a bone substitute in the jaws. Biomaterials. 1981; 2:19–22.11. Daculsi G, LeGeros RZ, Nery E, Lynch K, Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989; 23:883–894.12. Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol. 1992; 63:729–735.13. Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials. 1998; 19:133–139.14. Daculsi G, Passuti N, Martin S, Deudon C, Legeros RZ, Raher S. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J Biomed Mater Res. 1990; 24:379–396.15. Jiang G, Shi D. Coating of hydroxyapatite on highly porous Al2O3 substrate for bone substitutes. J Biomed Mater Res. 1998; 43:77–81.16. Pae A, Lee H, Kim HS, Baik J, Woo YH. Cellular attachment and gene expression of osteoblast-like cells on zirconia ceramic surfaces. J Korean Acad Prosthodont. 2008; 46:227–237.17. Guazzato M, Quach L, Albakry M, Swain MV. Influence of surface and heat treatments on the flexural strength of Y-TZP dental ceramic. J Dent. 2005; 33:9–18.18. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999; 20:1–25.19. Piconi C, Burger W, Richter HG, Cittadini A, Maccauro G, Covacci V, Bruzzese N, Ricci GA, Marmo E. Y-TZP ceramics for artificial joint replacements. Biomaterials. 1998; 19:1489–1494.20. Sollazzo V, Pezzetti F, Scarano A, Piattelli A, Bignozzi CA, Massari L, Brunelli G, Carinci F. Zirconium oxide coating improves implant osseointegration in vivo. Dent Mater. 2008; 24:357–361.21. Schultze-Mosgau S, Schliephake H, Radespiel-Tröger M, Neukam FW. Osseointegration of endodontic endosseous cones: zirconium oxide vs titanium. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 89:91–98.22. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986; 1:11–25.23. Andreiotelli M, Wenz HJ, Kohal RJ. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin Oral Implants Res. 2009; 20:32–47.24. McKinney RV Jr, Steflik DE, Koth DL. The biologic response to the single-crystal sapphire endosteal dental implant: scanning electron microscopic observations. J Prosthet Dent. 1984; 51:372–379.25. Vagkopoulou T, Koutayas SO, Koidis P, Strub JR. Zirconia in dentistry: Part 1. Discovering the nature of an upcoming bioceramic. Eur J Esthet Dent. 2009; 4:130–151.26. Wohlfahrt JC, Lyngstadaas SP, Rønold HJ, Saxegaard E, Ellingsen JE, Karlsson S, Aass AM. Porous titanium granules in the surgical treatment of peri-implant osseous defects: a randomized clinical trial. Int J Oral Maxillofac Implants. 2012; 27:401–410.27. Steinemann SG. Titanium--the material of choice? Periodontol 2000. 1998; 17:7–21.28. Langhoff JD, Voelter K, Scharnweber D, Schnabelrauch M, Schlottig F, Hefti T, Kalchofner K, Nuss K, von Rechenberg B. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: a study in sheep. Int J Oral Maxillofac Surg. 2008; 37:1125–1132.29. Itälä AI, Ylänen HO, Ekholm C, Karlsson KH, Aro HT. Pore diameter of more than 100 microm is not requisite for bone ingrowth in rabbits. J Biomed Mater Res. 2001; 58:679–683.30. Aboushelib MN, Salem NA, Taleb AL, El Moniem NM. Influence of surface nano-roughness on osseointegration of zirconia implants in rabbit femur heads using selective infiltration etching technique. J Oral Implantol. 2013; 39:583–590.31. Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983; 96:191–198.32. Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999; 14:893–903.33. Pae A, Lee H, Kim HS, Kwon YD, Woo YH. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed Mater. 2009; 4:025005.34. Tai G, Christodoulou I, Bishop AE, Polak JM. Use of green fluorescent fusion protein to track activation of the transcription factor osterix during early osteoblast differentiation. Biochem Biophys Res Commun. 2005; 333:1116–1122.35. Franceschi RT, Xiao G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003; 88:446–454.36. Barrère F, van der Valk CM, Dalmeijer RA, Meijer G, van Blitterswijk CA, de Groot K, Layrolle P. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. J Biomed Mater Res A. 2003; 66:779–788.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of a Hydroxyapatite and Tricalcium - Phosphate Coating on Titanium Fiber - Mesh Stem
- Minimum Five Year Follow-Up Study of Porous-Coated Versus Hydroxyapatite Porous-Coated Cementless Total Hip Replacement
- Study about Biodegradation of Hydroxyapatite and beta-tricalcium Phosphate Coating Layer
- Teh Effect of Hydroxyapatite Coating on the Mechanical Strengths and Histologic Profiles of Porous Titanium Implants in Dogs
- Effects of different sizes of Hydroxyapatite/beta-Tricalcium phosphate particles on vertical bone augmentation